A vibrational spectral maker for probing the hydrogen-bonding status of protonated Asp and Glu residues
- PMID: 15653739
- PMCID: PMC1305378
- DOI: 10.1529/biophysj.104.047639
A vibrational spectral maker for probing the hydrogen-bonding status of protonated Asp and Glu residues
Abstract
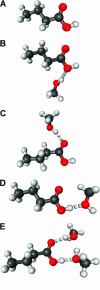
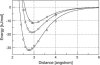
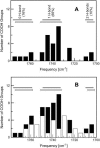
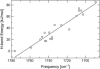
Hydrogen bonding is a fundamental element in protein structure and function. Breaking a single hydrogen bond may impair the stability of a protein. We report an infrared vibrational spectral marker for probing the hydrogen-bond number for buried, protonated Asp or Glu residues in proteins. Ab initio computational studies were performed on hydrogen-bonding interactions of a COOH group with a variety of side-chain model compounds of polar and charged amino acids in vacuum using density function theory. For hydrogen-bonding interactions with polar side-chain groups, our results show a strong correlation between the C=O stretching frequency and the hydrogen bond number of a COOH group: approximately 1759-1776 cm(-1) for zero, approximately 1733-1749 cm(-1) for one, and 1703-1710 cm(-1) for two hydrogen bonds. Experimental evidence for this correlation will be discussed. In addition, we show an approximate linear correlation between the C=O stretching frequency and the hydrogen-bond strength. We propose that a two-dimensional infrared spectroscopy, C=O stretching versus O-H stretching, may be employed to identify the specific type of hydrogen-bonding interaction. This vibrational spectral marker for hydrogen-bonding interaction is expected to enhance the power of time-resolved Fourier transform infrared spectroscopy for structural characterization of functionally important intermediates of proteins.
Figures

References
-
- Acharya, S., and S. S. Karnik. 1996. Modulation of GDP release from transducin by the conserved Glu134-Arg135 sequence in rhodopsin. J. Biol. Chem. 271:25406–25411. - PubMed
-
- Arnis, S., K. Fahmy, K. P. Hofmann, and T. P. Sakmar. 1994. A conserved carboxylic acid group mediates light-dependent proton uptake and signaling by rhodopsin. J. Biol. Chem. 269:23879–23881. - PubMed
-
- Barber-Armstrong, W., T. Donaldson, H. Wijesooriya, R. A. Silva, and S. M. Decatur. 2004. Empirical relationships between isotope-edited IR spectra and helix geometry in model peptides. J. Am. Chem. Soc. 126:2339–2345. - PubMed
-
- Barth, A. 2000. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Bio. 74:141–173. - PubMed
-
- Bergo, V., E. N. Spudich, J. Spudich, and K. J. Rothschild. 2003. Conformational changes detected in a sensory rhodopsin II-transducer complex. J. Biol. Chem. 278:36556–36562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

