Elicitation of allergic asthma by immunoglobulin free light chains
- PMID: 15653775
- PMCID: PMC547820
- DOI: 10.1073/pnas.0406808102
Elicitation of allergic asthma by immunoglobulin free light chains
Abstract
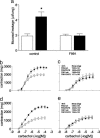
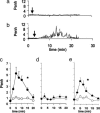
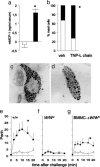
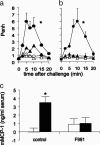
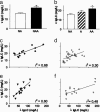
The observation that only 50% of patients with adult asthma manifest atopy indicates that other inflammatory mechanisms are likely involved in producing the characteristic features of this disorder; namely reversible airway obstruction, hyperresponsiveness, and pulmonary inflammation. Our recent discovery that antigen-specific Ig free light chains (LCs) mediate hypersensitivity-like responses suggests that these molecules may be of import in the pathophysiology of asthma. Using a murine experimental model of nonatopic asthma, we now have shown that an LC antagonist, the 9-mer peptide F991, can abrogate the development of airway obstruction, hyperresponsiveness, and pulmonary inflammation. Further, passive immunization with antigen-specific LCs and subsequent airway challenge can elicit a mast cell-dependent reaction leading to acute bronchoconstriction. These findings, and the demonstration that the concentration of free kappa LCs in the sera of patients with adult asthma were significantly increased (as compared with age-matched nonasthmatic individuals), provide previously undescribed insight into the pathogenesis of asthma. In addition, the ability to inhibit pharmacologically LC-induced mast cell activation provides a therapeutic means to prevent or ameliorate the adverse bronchopulmonary manifestations of this incapacitating disorder.
Figures
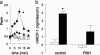
Comment in
-
A new mechanism for immunologic initiation of asthma.Proc Natl Acad Sci U S A. 2005 Feb 1;102(5):1267-8. doi: 10.1073/pnas.0409828102. Epub 2005 Jan 26. Proc Natl Acad Sci U S A. 2005. PMID: 15677319 Free PMC article. No abstract available.
Similar articles
-
Key role for mast cells in nonatopic asthma.J Immunol. 2002 Aug 15;169(4):2044-53. doi: 10.4049/jimmunol.169.4.2044. J Immunol. 2002. PMID: 12165531
-
Ig-free light chains play a crucial role in murine mast cell-dependent colitis and are associated with human inflammatory bowel diseases.J Immunol. 2010 Jul 1;185(1):653-9. doi: 10.4049/jimmunol.0901129. Epub 2010 May 26. J Immunol. 2010. PMID: 20505143
-
Topical application of F991, an immunoglobulin free light chain antagonist, prevents development of contact sensitivity in mice.Clin Exp Allergy. 2007 Feb;37(2):270-5. doi: 10.1111/j.1365-2222.2007.02655.x. Clin Exp Allergy. 2007. PMID: 17250700
-
Free immunoglobulin light chains as target in the treatment of chronic inflammatory diseases.Eur J Pharmacol. 2006 Mar 8;533(1-3):319-26. doi: 10.1016/j.ejphar.2005.12.065. Epub 2006 Feb 7. Eur J Pharmacol. 2006. PMID: 16455071 Review.
-
Immunoglobulin Free Light Chains in the Pathogenesis of Lung Disorders.Iran J Allergy Asthma Immunol. 2017 Aug;16(4):282-288. Iran J Allergy Asthma Immunol. 2017. PMID: 28865407 Review.
Cited by
-
Asthma: eosinophil disease, mast cell disease, or both?Allergy Asthma Clin Immunol. 2008 Jun 15;4(2):84-90. doi: 10.1186/1710-1492-4-2-84. Epub 2008 Jun 15. Allergy Asthma Clin Immunol. 2008. PMID: 20525129 Free PMC article.
-
Human umbilical cord blood-derived mast cells: a unique model for the study of neuro-immuno-endocrine interactions.Stem Cell Rev. 2006;2(2):143-54. doi: 10.1007/s12015-006-0021-z. Stem Cell Rev. 2006. PMID: 17237553 Review.
-
Mass spectrometry-based metabolomics for irritable bowel syndrome biomarkers.Therap Adv Gastroenterol. 2019 Nov 7;12:1756284819886425. doi: 10.1177/1756284819886425. eCollection 2019. Therap Adv Gastroenterol. 2019. PMID: 35154385 Free PMC article.
-
A new mechanism for immunologic initiation of asthma.Proc Natl Acad Sci U S A. 2005 Feb 1;102(5):1267-8. doi: 10.1073/pnas.0409828102. Epub 2005 Jan 26. Proc Natl Acad Sci U S A. 2005. PMID: 15677319 Free PMC article. No abstract available.
-
Polyclonal free light chains: a biomarker of inflammatory disease or treatment target?F1000 Med Rep. 2013;5:4. doi: 10.3410/M5-4. Epub 2013 Feb 1. F1000 Med Rep. 2013. PMID: 23413370 Free PMC article.
References
-
- Holgate, S. T., Godfrey, R. C. & Church, M. K. (1993) in Allergy, Principles and Practice, eds. Middleton, E., Reed, C. E., Ellis, E. F., Atkinson, N. F., Yuniger, J. W. & Busse, W. W. (Mosby, St. Louis), pp. 267-301.
-
- Galli, S. J. & Costa, J. J. (1995) Allergy 50, 851-862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

