Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis
- PMID: 15653820
- PMCID: PMC544180
- DOI: 10.1128/CMR.18.1.81-101.2005
Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis
Abstract
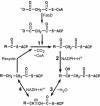
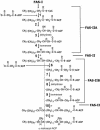
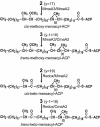
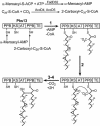
Mycobacterium tuberculosis is known to synthesize alpha-, methoxy-, and keto-mycolic acids. We propose a detailed pathway to the biosynthesis of all mycolic acids in M. tuberculosis. Fatty acid synthetase I provides C(20)-S-coenzyme A to the fatty acid synthetase II system (FAS-IIA). Modules of FAS-IIA and FAS-IIB introduce cis unsaturation at two locations on a growing meroacid chain to yield three different forms of cis,cis-diunsaturated fatty acids (intermediates to alpha-, methoxy-, and keto-meroacids). These are methylated, and the mature meroacids and carboxylated C(26)-S-acyl carrier protein enter into the final Claisen-type condensation with polyketide synthase-13 (Pks13) to yield mycolyl-S-Pks13. We list candidate genes in the genome encoding the proposed dehydrase and isomerase in the FAS-IIA and FAS-IIB modules. We propose that the processing of mycolic acids begins by transfer of mycolic acids from mycolyl-S-Pks13 to d-mannopyranosyl-1-phosphoheptaprenol to yield 6-O-mycolyl-beta-d-mannopyranosyl-1-phosphoheptaprenol and then to trehalose 6-phosphate to yield phosphorylated trehalose monomycolate (TMM-P). Phosphatase releases the phosphate group to yield TMM, which is immediately transported outside the cell by the ABC transporter. Antigen 85 then catalyzes the transfer of a mycolyl group from TMM to the cell wall arabinogalactan and to other TMMs to produce arabinogalactan-mycolate and trehalose dimycolate, respectively. We list candidate genes in the genome that encode the proposed mycolyltransferases I and II, phosphatase, and ABC transporter. The enzymes within this total pathway are targets for new drug discovery.
Figures








References
-
- Admiraal, S. J., C. T. Walsh, and C. Khosla. 2001. The loading module of rifamycin synthetase is an adenylation-thiolation didomain with substrate tolerance for substituted benzoates. Biochemistry 40:6116-6123. - PubMed
-
- Anderson, D. H., G. Harth, M. A. Horwitz, and D. Eisenberg. 2001. An interfacial mechanism and a class of inhibitors inferred from two crystal structures of the Mycobacterium tuberculosis 30 kDa major secretory protein (antigen 85B), a mycolyl transferase. J. Mol. Biol. 307:671-681. - PubMed
-
- Asselineau, C., and J. Asselineau. 1978. Trehalose containing glycolipids. Prog. Chem. Fats Other Lipids 16:59-99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

