Exploiting T cell receptor genes for cancer immunotherapy
- PMID: 15654813
- PMCID: PMC1809284
- DOI: 10.1111/j.1365-2249.2005.02715.x
Exploiting T cell receptor genes for cancer immunotherapy
Abstract
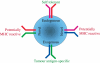
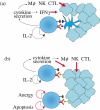
Adoptive antigen-specific immunotherapy is an attractive concept for the treatment of cancer because it does not require immunocompetence of patients, and the specificity of transferred lymphocytes can be targeted against tumour-associated antigens that are poorly immunogenic and thus fail to effectively trigger autologous T cell responses. As the isolation and in vitro expansion of antigen-specific lymphocytes is difficult, 'conventional' adoptive T cell therapy can only be carried out in specialized centres in small numbers of patients. However, T cell receptor (TCR) genes isolated from antigen-specific T cells can be exploited as generic therapeutic molecules for 'unconventional' antigen-specific immunotherapy. Retroviral TCR gene transfer into patient T cells can readily produce populations of antigen-specific lymphocytes after a single round of polyclonal T cell stimulation. TCR gene modified lymphocytes are functionally competent in vitro, and can have therapeutic efficacy in murine models in vivo. TCR gene expression is stable and modified lymphocytes can develop into memory T cells. Introduction of TCR genes into CD8(+) and CD4(+) lymphocytes provides an opportunity to use the same TCR specificity to produce antigen-specific killer and helper T lymphocytes. Thus, TCR gene therapy provides an attractive strategy to develop antigen-specific immunotherapy with autologous lymphocytes as a generic treatment option.
Figures

Similar articles
-
Human effector T cells derived from central memory cells rather than CD8(+)T cells modified by tumor-specific TCR gene transfer possess superior traits for adoptive immunotherapy.Cancer Lett. 2013 Oct 10;339(2):195-207. doi: 10.1016/j.canlet.2013.06.009. Epub 2013 Jun 18. Cancer Lett. 2013. PMID: 23791878
-
Rapid alphabeta TCR-mediated responses in gammadelta T cells transduced with cancer-specific TCR genes.Gene Ther. 2009 May;16(5):620-8. doi: 10.1038/gt.2009.6. Epub 2009 Feb 26. Gene Ther. 2009. PMID: 19242528
-
HLA class II restricted T-cell receptor gene transfer generates CD4+ T cells with helper activity as well as cytotoxic capacity.Gene Ther. 2005 Dec;12(23):1686-95. doi: 10.1038/sj.gt.3302586. Gene Ther. 2005. PMID: 16034453
-
Adoptive immuno-gene therapy of cancer with single chain antibody [scFv(Ig)] gene modified T lymphocytes.J Biol Regul Homeost Agents. 2004 Apr-Jun;18(2):134-40. J Biol Regul Homeost Agents. 2004. PMID: 15471217 Review.
-
Generation of tumor-specific T-cell therapies.Blood Rev. 2006 Mar;20(2):61-9. doi: 10.1016/j.blre.2005.05.001. Epub 2005 Jun 22. Blood Rev. 2006. PMID: 15978709 Review.
Cited by
-
Generation of diffuse large B cell lymphoma-associated antigen-specific Vα6/Vβ13+T cells by TCR gene transfer.J Hematol Oncol. 2011 Jan 11;4:2. doi: 10.1186/1756-8722-4-2. J Hematol Oncol. 2011. PMID: 21223579 Free PMC article.
-
A GMP-compliant protocol to expand and transfect cancer patient T cells with mRNA encoding a tumor-specific chimeric antigen receptor.Cancer Immunol Immunother. 2014 Oct;63(10):999-1008. doi: 10.1007/s00262-014-1572-5. Epub 2014 Jun 18. Cancer Immunol Immunother. 2014. PMID: 24938475 Free PMC article.
-
Subtle affinity-enhancing mutations in a myelin oligodendrocyte glycoprotein-specific TCR alter specificity and generate new self-reactivity.J Immunol. 2009 Apr 1;182(7):4439-47. doi: 10.4049/jimmunol.0804377. J Immunol. 2009. PMID: 19299745 Free PMC article.
-
A new way to generate cytolytic tumor-specific T cells: electroporation of RNA coding for a T cell receptor into T lymphocytes.Cancer Immunol Immunother. 2006 Sep;55(9):1132-41. doi: 10.1007/s00262-005-0098-2. Epub 2005 Dec 13. Cancer Immunol Immunother. 2006. PMID: 16344988 Free PMC article.
-
The construction of chimeric T-Cell receptor with spacer base of modeling study of VHH and MUC1 interaction.J Biomed Biotechnol. 2011;2011:578128. doi: 10.1155/2011/578128. Epub 2011 Aug 22. J Biomed Biotechnol. 2011. PMID: 21869862 Free PMC article.
References
-
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–4. - PubMed
-
- Glennie MJ, van de Winkel JG. Renaissance of cancer therapeutic antibodies. Drug Discov Today. 2003;8:503–10. - PubMed
-
- Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–55. - PubMed
-
- Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

