Interactions between the mannose receptor and thyroid autoantigens
- PMID: 15654820
- PMCID: PMC1809290
- DOI: 10.1111/j.1365-2249.2004.02689.x
Interactions between the mannose receptor and thyroid autoantigens
Abstract
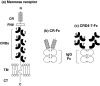
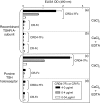
Thyroid autoantigens require internalization and processing by antigen-presenting cells to induce immune responses. Besides pinocytosis, antigen uptake can be receptor-mediated. The mannose receptor (ManR) has a cysteine rich domain (CR) and eight carbohydrate recognition domains (CRD) that bind glycosylated proteins. The TSH receptor (TSHR), thyroid peroxidase (TPO) and thyroglobulin (Tg) are glycoproteins. To investigate a role for the ManR in thyroid autoimmunity, we tested the interaction between these autoantigens and chimeric ManRs. Plasmids encoding the CR-domain linked to IgG-Fc (CR-Fc) and CDR domains 4-7 linked to IgG-Fc (CDR4-7-Fc) were expressed and purified with Protein A. Enzyme-linked immunosorbent assay (ELISA) plates were coated with human thyroid autoantigens and CR-Fc or CRD4-7-Fc binding detected with peroxidase-conjugated anti-IgG-Fc. CRD4-7-Fc binding was highest for the TSHR, followed by Tg and was minimal for TPO. CR-Fc bound to Tg but not to TSHR or TPO. The interaction between the TSHR and CRD-Fc was calcium-dependent; it was inhibited by mannose (not galactose), and required a glycosylated TSHR A-subunit. Moreover, precomplexing the TSHR A-subunit with CRD-Fc (but not CR-Fc), or adding mannose (but not galactose), decreased in vitro responses of splenocytes from TSHR-immunized mice. Our data indicate that the ManR may participate in autoimmune responses to Tg and the TSHR but not to TPO. Most important, ManR binding of heavily glycosylated TSHR A-subunits suggests a mechanism by which the minute amounts of A-subunit protein shed from the thyroid may be captured by antigen-presenting cells located in the gland or in draining lymph nodes.
Figures


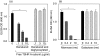

Similar articles
-
Induction of oral tolerance in human autoimmune thyroid disease.Thyroid. 1998 Mar;8(3):229-34. doi: 10.1089/thy.1998.8.229. Thyroid. 1998. PMID: 9545109 Clinical Trial.
-
Cytokines, IgG subclasses and costimulation in a mouse model of thyroid autoimmunity induced by injection of fibroblasts co-expressing MHC class II and thyroid autoantigens.Clin Exp Immunol. 2000 Nov;122(2):170-9. doi: 10.1046/j.1365-2249.2000.01362.x. Clin Exp Immunol. 2000. PMID: 11091271 Free PMC article.
-
Thyroid antigens, not central tolerance, control responses to immunization in BALB/c versus C57BL/6 mice.Thyroid. 2009 May;19(5):503-9. doi: 10.1089/thy.2008.0420. Thyroid. 2009. PMID: 19348579 Free PMC article.
-
Monoclonal antibodies to thyroid specific autoantigens.Autoimmunity. 2003 Sep-Nov;36(6-7):361-6. doi: 10.1080/08916930310001603055. Autoimmunity. 2003. PMID: 14669943 Review.
-
[Genes for autoantigens].Nihon Rinsho. 1999 Aug;57(8):1729-36. Nihon Rinsho. 1999. PMID: 10483241 Review. Japanese.
Cited by
-
An attempt to induce "Graves' disease of the gonads" by immunizing mice with the luteinizing hormone receptor provides insight into breaking tolerance to self-antigens.Thyroid. 2011 Jul;21(7):773-81. doi: 10.1089/thy.2010.0460. Epub 2011 Jun 7. Thyroid. 2011. PMID: 21649471 Free PMC article.
-
A role for exposed mannosylations in presentation of human therapeutic self-proteins to CD4+ T lymphocytes.Proc Natl Acad Sci U S A. 2007 May 22;104(21):8965-70. doi: 10.1073/pnas.0702120104. Epub 2007 May 14. Proc Natl Acad Sci U S A. 2007. PMID: 17502612 Free PMC article.
-
Glycosylation in Autoimmune Diseases.Adv Exp Med Biol. 2021;1325:205-218. doi: 10.1007/978-3-030-70115-4_10. Adv Exp Med Biol. 2021. PMID: 34495537
-
Glycosylation in the Thyroid Gland: Vital Aspects of Glycoprotein Function in Thyrocyte Physiology and Thyroid Disorders.Int J Mol Sci. 2018 Sep 17;19(9):2792. doi: 10.3390/ijms19092792. Int J Mol Sci. 2018. PMID: 30227620 Free PMC article. Review.
-
Review and hypothesis: does Graves' disease develop in non-human great apes?Thyroid. 2011 Dec;21(12):1359-66. doi: 10.1089/thy.2011.0209. Epub 2011 Nov 8. Thyroid. 2011. PMID: 22066476 Free PMC article. Review.
References
-
- Engering AJ, Cella M, Fluitsma D, et al. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27:2417–25. - PubMed
-
- Taylor ME, Conary JT, Lennartz MR, Stahl PD, Drickamer K. Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J Biol Chem. 1990;265:12156–62. - PubMed
-
- Leteux C, Chai W, Loveless RW, et al. The cysteine-rich domain of the macrophage mannose receptor is a multispecific lectin that recognizes chondroitin sulfates A and B and sulfated oligosaccharides of blood group Lewis (a) and Lewis (x) types in addition to the sulfated N-glycans of lutropin. J Exp Med. 2000;191:1117–26. - PMC - PubMed
-
- Taylor ME, Drickamer K. Structural requirements for high affinity binding of complex ligands by the macrophage mannose receptor. J Biol Chem. 1993;268:399–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

