Preclinical pharmacology of lumiracoxib: a novel selective inhibitor of cyclooxygenase-2
- PMID: 15655513
- PMCID: PMC1576032
- DOI: 10.1038/sj.bjp.0706078
Preclinical pharmacology of lumiracoxib: a novel selective inhibitor of cyclooxygenase-2
Abstract
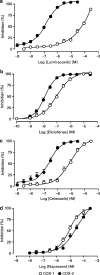
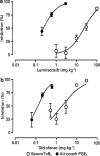
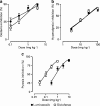
1. This manuscript presents the preclinical profile of lumiracoxib, a novel cyclooxygenase-2 (COX-2) selective inhibitor. 2. Lumiracoxib inhibited purified COX-1 and COX-2 with K(i) values of 3 and 0.06 microM, respectively. In cellular assays, lumiracoxib had an IC(50) of 0.14 microM in COX-2-expressing dermal fibroblasts, but caused no inhibition of COX-1 at concentrations up to 30 microM (HEK 293 cells transfected with human COX-1). 3. In a human whole blood assay, IC(50) values for lumiracoxib were 0.13 microM for COX-2 and 67 microM for COX-1 (COX-1/COX-2 selectivity ratio 515). 4. Lumiracoxib was rapidly absorbed following oral administration in rats with peak plasma levels being reached between 0.5 and 1 h. 5. Ex vivo, lumiracoxib inhibited COX-1-derived thromboxane B(2) (TxB(2)) generation with an ID(50) of 33 mg kg(-1), whereas COX-2-derived production of prostaglandin E(2) (PGE(2)) in the lipopolysaccharide-stimulated rat air pouch was inhibited with an ID(50) value of 0.24 mg kg(-1). 6. Efficacy of lumiracoxib in rat models of hyperalgesia, oedema, pyresis and arthritis was dose-dependent and similar to diclofenac. However, consistent with its low COX-1 inhibitory activity, lumiracoxib at a dose of 100 mg kg(-1) orally caused no ulcers and was significantly less ulcerogenic than diclofenac (P<0.05). 7. Lumiracoxib is a highly selective COX-2 inhibitor with anti-inflammatory, analgesic and antipyretic activities comparable with diclofenac, the reference NSAID, but with much improved gastrointestinal safety.
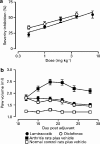
Figures


References
-
- ABBOTT LABORATORIES LIMITED 2002. Brufen 400 mg Tablets UK Summary of Product Characteristics, February 2002
-
- APPLETON I., TOMLINSON A., MITCHELL J.A., WILLOUGHBY D.A. Distribution of cyclooxygenase isoforms in murine chronic granulomatous inflammation. Implications for future anti-inflammatory therapy. J. Pathol. 1995;176:413–420. - PubMed
-
- BETTINI R., GROSSI E., RAPAZZINI P., GIARDINA G. Diclofenac sodium versus acetylsalicylic acid: a randomized study in febrile patients. J. Int. Med. Res. 1986;14:95–100. - PubMed
-
- BILLINGHAM M.E. Models of arthritis and the search for anti-arthritic drugs. Pharmacol. Ther. 1983;21:389–428. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

