The composition of the GABA receptor at the Caenorhabditis elegans neuromuscular junction
- PMID: 15655525
- PMCID: PMC1576029
- DOI: 10.1038/sj.bjp.0706052
The composition of the GABA receptor at the Caenorhabditis elegans neuromuscular junction
Abstract
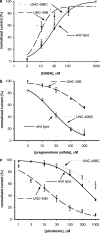
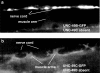
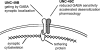
1. The unc-49 gene of the nematode Caenorhabditis elegans encodes three gamma-aminobutyric acid type A (GABA(A)) receptor subunits. Two of these, UNC-49B and UNC-49C, are expressed at high abundance and co-localize at the neuromuscular junction. 2. The UNC-49B subunit is sufficient to form a GABA(A) receptor in vitro and in vivo. Furthermore, all loss-of-function unc-49 alleles lack functional UNC-49B. No mutations specifically inactivate UNC-49C. Thus, UNC-49C appears to be dispensable for receptor function; however, UNC-49C has been conserved among different nematode species, suggesting it plays a necessary role. 3. To ascertain whether UNC-49C is part of the GABA(A) receptor in vivo, we performed patch-clamp electrophysiology on C. elegans muscle cells. Sensitivity to GABA, and to the antagonists picrotoxin and pregnenolone sulfate, matched the UNC-49B/C heteromer rather than the UNC-49B homomer, for both exogenous and synaptically-released GABA. 4. The synaptic localization of UNC-49C requires the presence of UNC-49B, indicative of a physical association between the two subunits in vivo. Thus, the in vivo receptor is an UNC-49B/C heteromer. 5. UNC-49C plays a negative modulatory role. Using the rapid ligand-exchange technique in vitro, we determined that UNC-49C causes accelerated receptor desensitization. Previously, UNC-49C was shown to reduce single-channel conductance in UNC-49B/C heteromers. Thus, the function of UNC-49B is to provide GABA responsiveness and localization to synapses, while the function of UNC-49C is to negatively modulate receptor function and precisely shape inhibitory postsynaptic currents.
Figures
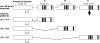


References
-
- ESSRICH C., LOREZ M., BENSON J.A., FRITSCHY J.M., LUSCHER B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1998;1:563–571. - PubMed
-
- FISHER J.L., ZHANG J., MACDONALD R.L. The role of alpha1 and alpha6 subtype amino-terminal domains in allosteric regulation of gamma-aminobutyric acid A receptors. Mol. Pharmacol. 1997;52:714–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

