A peptide corresponding to the neuropilin-1-binding site on VEGF(165) induces apoptosis of neuropilin-1-expressing breast tumour cells
- PMID: 15655556
- PMCID: PMC2361857
- DOI: 10.1038/sj.bjc.6602308
A peptide corresponding to the neuropilin-1-binding site on VEGF(165) induces apoptosis of neuropilin-1-expressing breast tumour cells
Abstract
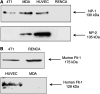
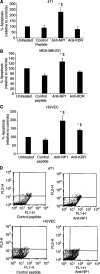
There is increasing evidence that vascular endothelial growth factor (VEGF) has autocrine as well as paracrine functions in tumour biology. Vascular endothelial growth factor-mediated cell survival signalling occurs via the classical tyrosine kinase receptors Flt-1, KDR/Flk-1 and the more novel neuropilin (NP) receptors, NP-1 and NP-2. A 24-mer peptide, which binds to neuropilin-1, induced apoptosis of murine and human breast carcinoma cells, whereas a peptide directed against KDR had no effect. Both anti-NP1 and anti-KDR peptides induced endothelial cell apoptosis. Confocal microscopy using 5-(6)-carboxyfluorescein-labelled peptides showed that anti-NP1 bound to both tumour and endothelial cells, whereas anti-KDR bound endothelial cells only. This study demonstrates that NP-1 plays an essential role in autocrine antiapoptotic signalling by VEGF in tumour cells and that NP1-blockade induces tumour cell and endothelial cell apoptosis. Specific peptides can therefore be used to target both autocrine (tumour cells) and paracrine (endothelial cells) signalling by VEGF.
Figures


References
-
- Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM (2001) Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res 61: 5736–5740 - PubMed
-
- Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM (2003) Competing autocrine pathways involving alternative neuropilin-1 ligands regulates chemotaxis of carcinoma cells. Cancer Res 63: 5230–5233 - PubMed
-
- Bachelder RE, Wendt MA, Mercurio AM (2002) Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res 62: 7203–7206 - PubMed
-
- Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nat Med 1: 27–30 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

