The Cys214-->Ser mutation in peripherin/rds causes a loss-of-function phenotype in transgenic mice
- PMID: 15656787
- PMCID: PMC1138968
- DOI: 10.1042/BJ20041960
The Cys214-->Ser mutation in peripherin/rds causes a loss-of-function phenotype in transgenic mice
Abstract
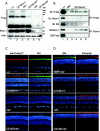
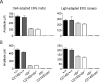
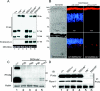
P/rds (peripherin/retinal degeneration slow) is a photoreceptor-specific membrane glycoprotein necessary for outer segment disc morphogenesis. Mutations in P/rds are associated with different blinding diseases. A C214S (Cys214-->Ser) missense mutation has been shown to be the cause for a late-onset form of ADRP (autosomal dominant retinitis pigmentosa) in humans. In the present study, we generated transgenic mice expressing P/rds with the C214S mutation and crossed them into rds mutant mice to elucidate the mechanism underlying the pathology of ADRP. Although an ample amount of transgene message was formed in C214S retinas from all transgenic lines, only a trace amount of the mutant protein was detected by Western blotting and immunoprecipitation. C214S mice on the wild-type or rds+/- backgrounds exhibited no signs of negative effects of the mutation on retinal structure or function, suggesting a loss-of-function phenotype. This phenotype is further supported by the absence of outer segment formation in the C214S mice on the rds-/- background. In contrast, expression of C214S protein in the inner retinal cells of transgenic mice or in COS cells resulted in the formation of a substantial amount of mutant protein, signifying a possible photoreceptor-specific regulation of P/rds. These results provide evidence that the loss-of-function phenotype seen in C214S transgenic mice shows a disease progression that correlates with ADRP patients carrying the same mutation, indicating that the C214S mutation on one allele of P/rds results in haploinsufficiency.
Figures



Similar articles
-
An intramembrane glutamic acid governs peripherin/rds function for photoreceptor disk morphogenesis.Invest Ophthalmol Vis Sci. 2007 Jul;48(7):2975-86. doi: 10.1167/iovs.07-0049. Invest Ophthalmol Vis Sci. 2007. PMID: 17591862
-
Cysteine residues of photoreceptor peripherin/rds: role in subunit assembly and autosomal dominant retinitis pigmentosa.Biochemistry. 1998 Jan 13;37(2):680-5. doi: 10.1021/bi972036i. Biochemistry. 1998. PMID: 9425091
-
Genetic supplementation of RDS alleviates a loss-of-function phenotype in C214S model of retinitis pigmentosa.Adv Exp Med Biol. 2008;613:129-38. doi: 10.1007/978-0-387-74904-4_14. Adv Exp Med Biol. 2008. PMID: 18188937 Free PMC article. No abstract available.
-
Role of subunit assembly in autosomal dominant retinitis pigmentosa linked to mutations in peripherin 2.Novartis Found Symp. 2004;255:95-112; discussion 113-6, 177-8. Novartis Found Symp. 2004. PMID: 14750599 Review.
-
[A molecular biological study on retinitis pigmentosa].Nippon Ganka Gakkai Zasshi. 1993 Dec;97(12):1394-405. Nippon Ganka Gakkai Zasshi. 1993. PMID: 7904791 Review. Japanese.
Cited by
-
ROM1 is redundant to PRPH2 as a molecular building block of photoreceptor disc rims.bioRxiv [Preprint]. 2023 Aug 29:2023.07.02.547380. doi: 10.1101/2023.07.02.547380. bioRxiv. 2023. Update in: Elife. 2023 Nov 22;12:RP89444. doi: 10.7554/eLife.89444. PMID: 37693615 Free PMC article. Updated. Preprint.
-
Ablation of the riboflavin-binding protein retbindin reduces flavin levels and leads to progressive and dose-dependent degeneration of rods and cones.J Biol Chem. 2017 Dec 22;292(51):21023-21034. doi: 10.1074/jbc.M117.785105. Epub 2017 Oct 27. J Biol Chem. 2017. PMID: 29079576 Free PMC article.
-
Gene therapy for PRPH2-associated ocular disease: challenges and prospects.Cold Spring Harb Perspect Med. 2014 Aug 28;4(11):a017376. doi: 10.1101/cshperspect.a017376. Cold Spring Harb Perspect Med. 2014. PMID: 25167981 Free PMC article. Review.
-
The K153Del PRPH2 mutation differentially impacts photoreceptor structure and function.Hum Mol Genet. 2016 Aug 15;25(16):3500-3514. doi: 10.1093/hmg/ddw193. Epub 2016 Jun 29. Hum Mol Genet. 2016. PMID: 27365499 Free PMC article.
-
Peripherin-2 and Rom-1 have opposing effects on rod outer segment targeting of retinitis pigmentosa-linked peripherin-2 mutants.Sci Rep. 2017 May 24;7(1):2321. doi: 10.1038/s41598-017-02514-5. Sci Rep. 2017. PMID: 28539581 Free PMC article.
References
-
- Molday R. S., Hicks D., Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest. Ophthalmol. Vis. Sci. 1987;28:50–61. - PubMed
-
- Connell G. J., Molday R. S. Molecular cloning, primary structure, and orientation of the vertebrate photoreceptor cell protein peripherin in the rod outer segment disk membrane. Biochemistry. 1990;29:4691–4698. - PubMed
-
- van Nie R., Ivanyi D., Demant P. A new H-2-linked mutation, rds, causing retinal degeneration in the mouse. Tissue Antigens. 1978;12:106–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

