Role of cis-acting elements in the control of SERCA2b Ca2+ pump mRNA decay by nuclear proteins
- PMID: 15656788
- PMCID: PMC1186718
- DOI: 10.1042/BJ20041568
Role of cis-acting elements in the control of SERCA2b Ca2+ pump mRNA decay by nuclear proteins
Abstract
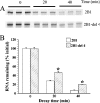
Alternative splicing at position 3495 b yields SERCA2 (sarco/endoplasmic reticulum Ca2+ pump 2) RNA species, namely SERCA2a and SERCA2b which differ in 3'-end regions. This results in SERCA2b RNA being less stable. In vitro decay experiments show that, in the presence of protein extracts from nuclei of LVMs (left ventricular myocytes), the rate of decay of both SERCA2b RNA and synthetic RNA from its 3'-region is greater than that of the corresponding SERCA2a RNA. To search for cis-acting instability elements in the 3'-region of SERCA2b, we examined the effects of LVM nuclear protein extracts on the in vitro decay of six short overlapping capped [m7G(5')ppp(5')Gm] and polyadenylated (A40) RNA fragments from the 3'-end region (3444-4472) of SERCA2b. The proximal fragment 2B1 (3444-3753) was the most unstable. 2B1 RNA without a cap or a polyadenylated tail was analysed further in electrophoretic mobility-shift assays, and was observed to bind to protein(s) in the nuclear extracts. Based on competition for binding to nuclear proteins between radiolabelled 2B1 RNA and short unlabelled RNA fragments, the cis-acting element involved in this binding was the sequence 2B1-4. 2B1-4 is a 35-base (3521-3555, CCAGUCCUGCUCGUUGUGGGCGUGCACCGAGGGGG) GC-rich region just past the splice site (3495). Nuclear extracts decreased the electrophoretic mobility of the radiolabelled 2B1-4 RNA which bound to two proteins (19 and 21 kDa) in cross-linking experiments. Excess 2B1-4 RNA decreased the decay of the 2B1 RNA by the nuclear protein extract. 2B1-del 4 RNA (2B1 with the 2B1-4 domain deleted) also decayed more slowly than the control 2B1 RNA. Thus SERCA2b contains a novel GC-rich cis-acting element involved in its decay by nuclear proteins.
Figures





Similar articles
-
Control of SERCA2a Ca2+ pump mRNA stability by nuclear proteins: role of domains in the 3'-untranslated region.Cell Calcium. 2005 Jan;37(1):17-24. doi: 10.1016/j.ceca.2004.06.003. Cell Calcium. 2005. PMID: 15541460
-
Characterization of SERCA2b Ca2+-Mg2+ ATPase mRNA decay by nuclear proteins.Cell Calcium. 2007 Jun;41(6):581-92. doi: 10.1016/j.ceca.2006.10.008. Epub 2006 Dec 1. Cell Calcium. 2007. PMID: 17141309
-
Sarcoplasmic reticulum Ca2+ pump mRNA stability in cardiac and smooth muscle: role of poly A+ tail length.Cell Calcium. 2004 May;35(5):479-84. doi: 10.1016/j.ceca.2003.12.001. Cell Calcium. 2004. PMID: 15003857
-
Control of protein expression through mRNA stability in calcium signalling.Cell Calcium. 2006 Oct;40(4):329-46. doi: 10.1016/j.ceca.2006.04.004. Epub 2006 Jun 12. Cell Calcium. 2006. PMID: 16765440 Review.
-
RNA-protein interactions and control of mRNA stability in neurons.J Neurosci Res. 2008 Feb 15;86(3):481-9. doi: 10.1002/jnr.21473. J Neurosci Res. 2008. PMID: 17853436 Review.
Cited by
-
Lung Beractant Increases Free Cytosolic Levels of Ca2+ in Human Lung Fibroblasts.PLoS One. 2015 Jul 31;10(7):e0134564. doi: 10.1371/journal.pone.0134564. eCollection 2015. PLoS One. 2015. PMID: 26230503 Free PMC article.
-
Calcium regulation by SERC-A before and during Alzheimer disease.Biomedica. 2023 Mar 30;43(1):51-60. doi: 10.7705/biomedica.6704. Biomedica. 2023. PMID: 37167461 Free PMC article. English, Spanish.
-
RNA binding proteins as mediators of pathological cardiac remodeling.Front Cell Dev Biol. 2024 May 16;12:1368097. doi: 10.3389/fcell.2024.1368097. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38818408 Free PMC article. Review.
References
-
- Clapham D. E. Calcium signaling. Cell. 1995;80:259–268. - PubMed
-
- East J. M. Sarco(endo)plasmic reticulum calcium pumps: recent advances in our understanding of structure/function and biology. Mol. Membr. Biol. 2000;17:189–200. - PubMed
-
- Misquitta C. M., Mack D. P., Grover A. K. Sarco/endoplasmic reticulum Ca2+ (SERCA)-pumps: link to heart beats and calcium waves. Cell Calcium. 1999;25:277–290. - PubMed
-
- Eggermont J. A., Wuytack F., Casteels R. Characterization of the 3′ end of the pig sarcoplasmic/endoplasmic-reticulum Ca2+ pump gene 2. Biochim. Biophys. Acta. 1991;1088:448–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

