Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides
- PMID: 15656908
- PMCID: PMC548510
- DOI: 10.1186/1742-4690-2-2
Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides
Abstract
Background: The antibacterial activity of host defense peptides (HDP) is largely mediated by permeabilization of bacterial membranes. The lipid membrane of enveloped viruses might also be a target of antimicrobial peptides. Therefore, we screened a panel of naturally occurring HDPs representing different classes for inhibition of early, Env-independent steps in the HIV replication cycle. A lentiviral vector-based screening assay was used to determine the inhibitory effect of HDPs on early steps in the replication cycle and on cell metabolism.
Results: Human LL37 and porcine Protegrin-1 specifically reduced lentiviral vector infectivity, whereas the reduction of luciferase activities observed at high concentrations of the other HDPs is primarily due to modulation of cellular activity and/ or cytotoxicity rather than antiviral activity. A retroviral vector was inhibited by LL37 and Protegrin-1 to similar extent, while no specific inhibition of adenoviral vector mediated gene transfer was observed. Specific inhibitory effects of Protegrin-1 were confirmed for wild type HIV-1.
Conclusion: Although Protegrin-1 apparently inhibits an early step in the HIV-replication cycle, cytotoxic effects might limit its use as an antiviral agent unless the specificity for the virus can be improved.
Figures
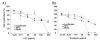

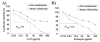

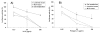

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

