Metabolizing enzyme toxicology assay chip (MetaChip) for high-throughput microscale toxicity analyses
- PMID: 15657119
- PMCID: PMC545843
- DOI: 10.1073/pnas.0406755102
Metabolizing enzyme toxicology assay chip (MetaChip) for high-throughput microscale toxicity analyses
Abstract
The clinical progression of new chemical entities to pharmaceuticals remains hindered by the relatively slow pace of technology development in toxicology and clinical safety evaluation, particularly in vitro approaches, that can be used in the preclinical and early clinical phases of drug development. To alleviate this bottle-neck, we have developed a metabolizing enzyme toxicology assay chip (MetaChip) that combines high-throughput P450 catalysis with cell-based screening on a microscale platform. The MetaChip concept is demonstrated by using sol-gel encapsulated P450s to activate the prodrug cyclophosphamide, which is the major constituent of the anticancer drug Cytoxan, as well as other compounds that are activated by P450 metabolism. The MetaChip provides a high-throughput microscale alternative to currently used in vitro methods for human metabolism and toxicology screening based on liver slices, cultured human hepatocytes, purified microsomal preparations, or isolated and purified P450s. This technology creates opportunities for rapid and inexpensive assessment of ADME/Tox (absorption, distribution, metabolism, excretion/toxicology) at very early phases of drug development, thereby enabling unsuitable candidates to be eliminated from consideration much earlier in the drug discovery process.
Figures
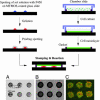



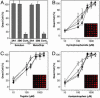
References
-
- Geysen, H. M., Schoenen, F., Wagner, D. & Wagner, R. (2003) Nat. Rev. Drug Discov. 2, 222–230. - PubMed
-
- Myers, P. L. (1997) Curr. Opin. Biotechnol. 2, 701–707. - PubMed
-
- Bleicher, K. H., Bohm, H. J., Muller, K. & Alanine, A.I. (2003) Nat. Rev. Drug Discov. 2, 369–378. - PubMed
-
- Petricoin, E. F., Zoon, K. C., Kohn, E. C., Barrett, J. C. & Liotta, L. A. (2002) Nat. Rev. Drug Discov. 1, 683–695. - PubMed
-
- Gombar, V. K., Silver, L. S. & Zhao, Z. (2003) Top. Med. Chem. 3, 1205–1225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

