Glutamate regulation of DARPP-32 phosphorylation in neostriatal neurons involves activation of multiple signaling cascades
- PMID: 15657149
- PMCID: PMC545831
- DOI: 10.1073/pnas.0409138102
Glutamate regulation of DARPP-32 phosphorylation in neostriatal neurons involves activation of multiple signaling cascades
Abstract
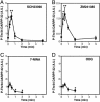
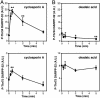
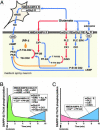
Dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) plays a central role in medium spiny neurons in the neostriatum in the integration of various neurotransmitter signaling pathways. In its Thr-34-phosphorylated form, it acts as a potent protein phosphatase-1 inhibitor, and, in its Thr-75-phosphorylated form, it acts as a cAMP-dependent kinase inhibitor. Here, we investigated glutamate-dependent signaling cascades in mouse neostriatal slices by analyzing the phosphorylation of DARPP-32 at Thr-34 and Thr-75. Treatment with glutamate (5 mM) caused a complex change in DARPP-32 Thr-34 phosphorylation. An initial rapid increase in Thr-34 phosphorylation was NMDA/alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/metabotropic glutamate-5 receptor-dependent and was mediated through activation of a neuronal nitric oxide synthase/nitric oxide/cGMP/cGMP-dependent kinase signaling cascade. A subsequent decrease in phosphorylation was attributable to activation of an NMDA/AMPA receptor/Ca2+/protein phosphatase-2B signaling cascade. This decrease was followed by rephosphorylation via a pathway involving metabotropic glutamate-5 receptor/phospholipase C and extracellular receptor kinase signaling cascade. Treatment with glutamate initially decreased Thr-75 phosphorylation through activation of NMDA/AMPA receptor/Ca2+/protein phosphatase-2A signaling. Thereafter, glutamate slowly increased Thr-75 phosphorylation through activation of metabotropic glutamate-1 receptor/phospholipase C signaling. Our analysis of DARPP-32 phosphorylation in the neostriatum revealed that glutamate activates at least five different signaling cascades with different time dependencies, resulting in complex regulation of protein kinase and protein phosphatase activities.
Figures



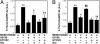
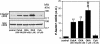
Similar articles
-
Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling.Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1322-7. doi: 10.1073/pnas.0237126100. Epub 2003 Jan 21. Proc Natl Acad Sci U S A. 2003. PMID: 12538871 Free PMC article.
-
Regulation of DARPP-32 dephosphorylation at PKA- and Cdk5-sites by NMDA and AMPA receptors: distinct roles of calcineurin and protein phosphatase-2A.J Neurochem. 2002 May;81(4):832-41. doi: 10.1046/j.1471-4159.2002.00876.x. J Neurochem. 2002. PMID: 12065642
-
Regulation of spinophilin Ser94 phosphorylation in neostriatal neurons involves both DARPP-32-dependent and independent pathways.J Neurochem. 2005 Dec;95(6):1642-52. doi: 10.1111/j.1471-4159.2005.03491.x. Epub 2005 Nov 21. J Neurochem. 2005. PMID: 16300646
-
DARPP-32 and modulation of cAMP signaling: involvement in motor control and levodopa-induced dyskinesia.Parkinsonism Relat Disord. 2004 Jul;10(5):281-6. doi: 10.1016/j.parkreldis.2004.02.010. Parkinsonism Relat Disord. 2004. PMID: 15196506 Review.
-
The role of DARPP-32 in the actions of drugs of abuse.Neuropharmacology. 2004;47 Suppl 1:14-23. doi: 10.1016/j.neuropharm.2004.05.010. Neuropharmacology. 2004. PMID: 15464122 Review.
Cited by
-
Nitric Oxide-Soluble Guanylyl Cyclase-Cyclic GMP Signaling in the Striatum: New Targets for the Treatment of Parkinson's Disease?Front Syst Neurosci. 2011 Jun 30;5:55. doi: 10.3389/fnsys.2011.00055. eCollection 2011. Front Syst Neurosci. 2011. PMID: 21747761 Free PMC article.
-
Adolescent psychosocial stress enhances sensitization to cocaine exposure in genetically vulnerable mice.Neurosci Res. 2020 Feb;151:38-45. doi: 10.1016/j.neures.2019.02.007. Epub 2019 Mar 1. Neurosci Res. 2020. PMID: 30831136 Free PMC article.
-
Mechanisms underlying the onset and expression of levodopa-induced dyskinesia and their pharmacological manipulation.J Neural Transm (Vienna). 2011 Dec;118(12):1661-90. doi: 10.1007/s00702-011-0698-2. Epub 2011 Sep 1. J Neural Transm (Vienna). 2011. PMID: 21881839 Review.
-
Reciprocal regulation of ARPP-16 by PKA and MAST3 kinases provides a cAMP-regulated switch in protein phosphatase 2A inhibition.Elife. 2017 Jun 14;6:e24998. doi: 10.7554/eLife.24998. Elife. 2017. PMID: 28613156 Free PMC article.
-
Facilitation of corticostriatal transmission following pharmacological inhibition of striatal phosphodiesterase 10A: role of nitric oxide-soluble guanylyl cyclase-cGMP signaling pathways.J Neurosci. 2015 Apr 8;35(14):5781-91. doi: 10.1523/JNEUROSCI.1238-14.2015. J Neurosci. 2015. PMID: 25855188 Free PMC article.
References
-
- Svenningsson, P., Nishi, A., Fisone, G., Girault, J. A., Nairn, A. C. & Greengard, P. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 269–296. - PubMed
-
- Fienberg, A. A., Hiroi, N., Mermelstein, P., Song, W.-J., Snyder, G. L., Nishi, A., Cheramy, A., O'Callaghan, J. P., Miller, D., Cole, D., et al. (1998) Science 281, 838–842. - PubMed
-
- Bibb, J. A., Snyder, G. L., Nishi, A., Yan, Z., Meijer, L., Fienberg, A. A., Tsai, L. H., Kwon, Y. T., Girault, J. A., Czernik, A. J., et al. (1999) Nature 402, 669–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

