PD166326, a novel tyrosine kinase inhibitor, has greater antileukemic activity than imatinib mesylate in a murine model of chronic myeloid leukemia
- PMID: 15657179
- PMCID: PMC1895078
- DOI: 10.1182/blood-2004-09-3534
PD166326, a novel tyrosine kinase inhibitor, has greater antileukemic activity than imatinib mesylate in a murine model of chronic myeloid leukemia
Abstract
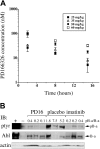
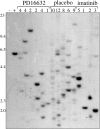
Imatinib mesylate is highly effective in newly diagnosed chronic myeloid leukemia (CML), but BCR/ABL (breakpoint cluster region/abelson murine leukemia)-positive progenitors persist in most patients with CML treated with imatinib mesylate, indicating the need for novel therapeutic approaches. In this study, we have used the murine CML-like myeloproliferative disorder as a platform to characterize the pharmacokinetic, signal transduction, and antileukemic properties of PD166326, one of the most potent members of the pyridopyrimidine class of protein tyrosine kinase inhibitors. In mice with the CML-like disease, PD166326 rapidly inhibited Bcr/Abl kinase activity after a single oral dose and demonstrated marked antileukemic activity in vivo. Seventy percent of PD166326-treated mice achieved a white blood cell (WBC) count less than 20.0 x 10(9)/L (20,000/microL) at necropsy, compared with only 8% of imatinib mesylate-treated animals. Further, two thirds of PD166326-treated animals had complete resolution of splenomegaly, compared with none of the imatinib mesylate-treated animals. Consistent with its more potent antileukemic effect in vivo, PD166326 was also superior to imatinib mesylate in inhibiting the constitutive tyrosine phosphorylation of numerous leukemia-cell proteins, including the src family member Lyn. PD166326 also prolonged the survival of mice with imatinib mesylate-resistant CML induced by the Bcr/Abl mutants P210/H396P and P210/M351T. Altogether, these findings demonstrate the potential of more potent Bcr/Abl inhibitors to provide more effective antileukemic activity. Clinical development of PD166326 or a related analog may lead to more effective drugs for the treatment of de novo and imatinib mesylate-resistant CML.
Figures
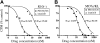




Similar articles
-
Inhibition of wild-type and mutant Bcr-Abl by AP23464, a potent ATP-based oncogenic protein kinase inhibitor: implications for CML.Blood. 2004 Oct 15;104(8):2532-9. doi: 10.1182/blood-2004-05-1851. Epub 2004 Jul 15. Blood. 2004. PMID: 15256422
-
Establishment of a murine model for therapy-treated chronic myelogenous leukemia using the tyrosine kinase inhibitor STI571.Blood. 2001 Nov 1;98(9):2808-16. doi: 10.1182/blood.v98.9.2808. Blood. 2001. PMID: 11675355
-
A cell-based screen for resistance of Bcr-Abl-positive leukemia identifies the mutation pattern for PD166326, an alternative Abl kinase inhibitor.Blood. 2005 Feb 15;105(4):1652-9. doi: 10.1182/blood-2004-06-2445. Epub 2004 Sep 30. Blood. 2005. PMID: 15459011
-
Overcoming kinase resistance in chronic myeloid leukemia.Int J Biochem Cell Biol. 2008;40(3):334-43. doi: 10.1016/j.biocel.2007.10.001. Int J Biochem Cell Biol. 2008. PMID: 18401881 Review.
-
Therapy options in imatinib failures.Oncologist. 2008 Apr;13(4):424-34. doi: 10.1634/theoncologist.2007-0170. Oncologist. 2008. PMID: 18448557 Review.
Cited by
-
An aging mouse model of human chronic myeloid leukemia.Oncogene. 2021 Apr;40(17):3152-3163. doi: 10.1038/s41388-021-01770-0. Epub 2021 Apr 6. Oncogene. 2021. PMID: 33824471 Free PMC article.
-
(124)I-iodopyridopyrimidinone for PET of Abl kinase-expressing tumors in vivo.J Nucl Med. 2010 Jan;51(1):121-9. doi: 10.2967/jnumed.109.066126. J Nucl Med. 2010. PMID: 20048131 Free PMC article.
-
Synthesis of 2-arylamino substituted 5,6-dihydropyrido[2,3-d]pyrimidine-7(8H)-ones from arylguanidines.Mol Divers. 2012 Nov;16(4):639-49. doi: 10.1007/s11030-012-9398-6. Epub 2012 Oct 10. Mol Divers. 2012. PMID: 23054532
-
Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F.PLoS One. 2006 Dec 20;1(1):e18. doi: 10.1371/journal.pone.0000018. PLoS One. 2006. PMID: 17183644 Free PMC article.
-
Contrastive learning in protein language space predicts interactions between drugs and protein targets.Proc Natl Acad Sci U S A. 2023 Jun 13;120(24):e2220778120. doi: 10.1073/pnas.2220778120. Epub 2023 Jun 8. Proc Natl Acad Sci U S A. 2023. PMID: 37289807 Free PMC article.
References
-
- O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348: 994-1004. - PubMed
-
- Garcia-Manero G, Faderl S, O'Brien S, Cortes J, Talpaz M, Kantarjian HM. Chronic myelogenous leukemia: a review and update of therapeutic strategies. Cancer. 2003;98: 437-457. - PubMed
-
- Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349: 1423-1432. - PubMed
-
- Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99: 1928-1937. - PubMed
-
- Kantarjian HM, O'Brien S, Cortes JE, et al. Treatment of Philadelphia chromosome-positive, accelerated-phase chronic myelogenous leukemia with imatinib mesylate. Clin Cancer Res. 2002;8: 2167-2176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

