New insights into the pathology of inherited cardiomyopathy
- PMID: 15657260
- PMCID: PMC1768710
- DOI: 10.1136/hrt.2004.040337
New insights into the pathology of inherited cardiomyopathy
Figures
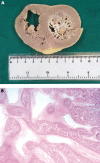


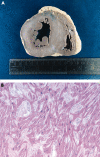
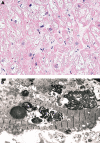
References
-
- Richardson P, McKenna WJ, Bristow M, et al. Report of the 1995 WHO/ISFC task force on the definition and classification of cardiomyopathies. Circulation 1996;93:841–42. - PubMed
-
- Towbin JA, Bowles NE. The failing heart. Nature 2002;415:227–33. ▸ An excellent up-to-date review of the identification of the genes responsible for cardiomyopathies providing further insights into the pathogenesis of these disorders. - PubMed
-
- Bowles NE, Bowles KR, Towbin JA. The “final common pathway” hypothesis and inherited cardiovascular disease. The role of cytoskeletal proteins in dilated cardiomyopathy. Herz 2000;25:168–75. - PubMed
-
- Sinagra G, Di Lenarda A, Brodsky GL, et al. Current perspective. New insights into the molecular basis of familial dilated cardiomyopathy. Ital Heart J 2001;2:280–6. ▸ A comprehensive review of the molecular genetic basis of FDCM. - PubMed
-
- Sedmera D, Pexieder T, Vuillemin M, et al. Developmental patterning of the myocardium. Anat Rec 2000;258:319–37. ▸ An excellent review article with stunning electron micrographs explaining developmental patterning of the myocardium. This article provides a unique insight into the pathology of isolated LVNC. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
