Preferential phosphorylation of R-domain Serine 768 dampens activation of CFTR channels by PKA
- PMID: 15657296
- PMCID: PMC2217491
- DOI: 10.1085/jgp.200409076
Preferential phosphorylation of R-domain Serine 768 dampens activation of CFTR channels by PKA
Abstract
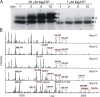
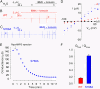
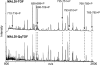
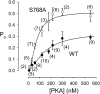
CFTR (cystic fibrosis transmembrane conductance regulator), the protein whose dysfunction causes cystic fibrosis, is a chloride ion channel whose gating is controlled by interactions of MgATP with CFTR's two cytoplasmic nucleotide binding domains, but only after several serines in CFTR's regulatory (R) domain have been phosphorylated by cAMP-dependent protein kinase (PKA). Whereas eight R-domain serines have previously been shown to be phosphorylated in purified CFTR, it is not known how individual phosphoserines regulate channel gating, although two of them, at positions 737 and 768, have been suggested to be inhibitory. Here we show, using mass spectrometric analysis, that Ser 768 is the first site phosphorylated in purified R-domain protein, and that it and five other R-domain sites are already phosphorylated in resting Xenopus oocytes expressing wild-type (WT) human epithelial CFTR. The WT channels have lower activity than S768A channels (with Ser 768 mutated to Ala) in resting oocytes, confirming the inhibitory influence of phosphoserine 768. In excised patches exposed to a range of PKA concentrations, the open probability (P(o)) of mutant S768A channels exceeded that of WT CFTR channels at all [PKA], and the half-maximally activating [PKA] for WT channels was twice that for S768A channels. As the open burst duration of S768A CFTR channels was almost double that of WT channels, at both low (55 nM) and high (550 nM) [PKA], we conclude that the principal mechanism by which phosphoserine 768 inhibits WT CFTR is by hastening the termination of open channel bursts. The right-shifted P(o)-[PKA] curve of WT channels might explain their slower activation, compared with S768A channels, at low [PKA]. The finding that phosphorylation kinetics of WT or S768A R-domain peptides were similar provides no support for an alternative explanation, that early phosphorylation of Ser 768 in WT CFTR might also impair subsequent phosphorylation of stimulatory R-domain serines. The observed reduced sensitivity to activation by [PKA] imparted by Ser 768 might serve to ensure activation of WT CFTR by strong stimuli while dampening responses to weak signals.
Figures




Similar articles
-
Functional roles of nonconserved structural segments in CFTR's NH2-terminal nucleotide binding domain.J Gen Physiol. 2005 Jan;125(1):43-55. doi: 10.1085/jgp.200409174. Epub 2004 Dec 13. J Gen Physiol. 2005. PMID: 15596536 Free PMC article.
-
Severed channels probe regulation of gating of cystic fibrosis transmembrane conductance regulator by its cytoplasmic domains.J Gen Physiol. 2000 Sep;116(3):477-500. doi: 10.1085/jgp.116.3.477. J Gen Physiol. 2000. PMID: 10962022 Free PMC article.
-
Deletion of phenylalanine 508 causes attenuated phosphorylation-dependent activation of CFTR chloride channels.J Physiol. 2000 May 1;524 Pt 3(Pt 3):637-48. doi: 10.1111/j.1469-7793.2000.00637.x. J Physiol. 2000. PMID: 10790148 Free PMC article.
-
Influence of phosphorylation by protein kinase A on CFTR at the cell surface and endoplasmic reticulum.Biochim Biophys Acta. 1999 Dec 6;1461(2):275-83. doi: 10.1016/s0005-2736(99)00163-7. Biochim Biophys Acta. 1999. PMID: 10581361 Review.
-
Regulation of the CFTR chloride channel from humans and sharks.J Exp Zool. 1996 Jul 1;275(4):283-91. doi: 10.1002/(SICI)1097-010X(19960701)275:4<283::AID-JEZ6>3.0.CO;2-L. J Exp Zool. 1996. PMID: 8759925 Review.
Cited by
-
State-dependent regulation of cystic fibrosis transmembrane conductance regulator (CFTR) gating by a high affinity Fe3+ bridge between the regulatory domain and cytoplasmic loop 3.J Biol Chem. 2010 Dec 24;285(52):40438-47. doi: 10.1074/jbc.M110.161497. Epub 2010 Oct 15. J Biol Chem. 2010. PMID: 20952391 Free PMC article.
-
VX-770-mediated potentiation of numerous human CFTR disease mutants is influenced by phosphorylation level.Sci Rep. 2019 Sep 17;9(1):13460. doi: 10.1038/s41598-019-49921-4. Sci Rep. 2019. PMID: 31530897 Free PMC article.
-
A cluster of inhibitory residues in the regulatory domain prevents activation of the cystic fibrosis transmembrane conductance regulator.J Biol Chem. 2025 May;301(5):108460. doi: 10.1016/j.jbc.2025.108460. Epub 2025 Mar 26. J Biol Chem. 2025. PMID: 40154618 Free PMC article.
-
Identification of a novel post-hydrolytic state in CFTR gating.J Gen Physiol. 2012 May;139(5):359-70. doi: 10.1085/jgp.201210789. Epub 2012 Apr 16. J Gen Physiol. 2012. PMID: 22508846 Free PMC article.
-
CFTR (ABCC7) is a hydrolyzable-ligand-gated channel.Pflugers Arch. 2007 Feb;453(5):693-702. doi: 10.1007/s00424-006-0140-z. Epub 2006 Sep 26. Pflugers Arch. 2007. PMID: 17021796 Review.
References
-
- Aleksandrov, L., A. Mengos, X. Chang, A. Aleksandrov, and J.R. Riordan. 2001. Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 276:12918–12923. - PubMed
-
- Aleksandrov, L., A.A. Aleksandrov, X.B. Chang, and J.R. Riordan. 2002. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J. Biol. Chem. 277:15419–15425. - PubMed
-
- Borchardt, R., J. Kole, and J.A. Cohn. 1996. Phosphorylation of CFTR Ser-737 by protein kinase A. Pediatr. Pulmonol. Suppl. 13:212.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

