Nuclear transport of single molecules: dwell times at the nuclear pore complex
- PMID: 15657394
- PMCID: PMC2171583
- DOI: 10.1083/jcb.200411005
Nuclear transport of single molecules: dwell times at the nuclear pore complex
Abstract
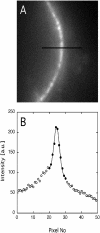
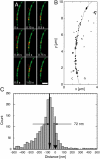
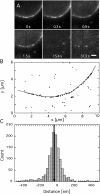
The mechanism by which macromolecules are selectively translocated through the nuclear pore complex (NPC) is still essentially unresolved. Single molecule methods can provide unique information on topographic properties and kinetic processes of asynchronous supramolecular assemblies with excellent spatial and time resolution. Here, single-molecule far-field fluorescence microscopy was applied to the NPC of permeabilized cells. The nucleoporin Nup358 could be localized at a distance of 70 nm from POM121-GFP along the NPC axis. Binding sites of NTF2, the transport receptor of RanGDP, were observed in cytoplasmic filaments and central framework, but not nucleoplasmic filaments of the NPC. The dwell times of NTF2 and transportin 1 at their NPC binding sites were 5.8 +/- 0.2 and 7.1 +/- 0.2 ms, respectively. Notably, the dwell times of these receptors were reduced upon binding to a specific transport substrate, suggesting that translocation is accelerated for loaded receptor molecules. Together with the known transport rates, our data suggest that nucleocytoplasmic transport occurs via multiple parallel pathways within single NPCs.
Figures
Similar articles
-
The nuclear pore component Nup358 promotes transportin-dependent nuclear import.J Cell Sci. 2009 Apr 15;122(Pt 8):1100-10. doi: 10.1242/jcs.040154. Epub 2009 Mar 19. J Cell Sci. 2009. PMID: 19299463
-
Computational and biochemical identification of a nuclear pore complex binding site on the nuclear transport carrier NTF2.J Mol Biol. 2004 Nov 19;344(2):303-10. doi: 10.1016/j.jmb.2004.09.043. J Mol Biol. 2004. PMID: 15522285
-
NLS-mediated NPC functions of the nucleoporin Pom121.FEBS Lett. 2010 Aug 4;584(15):3292-8. doi: 10.1016/j.febslet.2010.07.008. Epub 2010 Jul 14. FEBS Lett. 2010. PMID: 20624389
-
Structural biology of nucleocytoplasmic transport.Annu Rev Biochem. 2007;76:647-71. doi: 10.1146/annurev.biochem.76.052705.161529. Annu Rev Biochem. 2007. PMID: 17506639 Review.
-
Mechanisms of receptor-mediated nuclear import and nuclear export.Traffic. 2005 Mar;6(3):187-98. doi: 10.1111/j.1600-0854.2005.00270.x. Traffic. 2005. PMID: 15702987 Review.
Cited by
-
Surface decorated gold nanoparticles by linear and cyclic peptides as molecular transporters.Mol Pharm. 2013 Aug 5;10(8):3137-51. doi: 10.1021/mp400199e. Epub 2013 Jul 25. Mol Pharm. 2013. PMID: 23834324 Free PMC article.
-
Deciphering the intrinsically disordered characteristics of the FG-Nups through the lens of polymer physics.Nucleus. 2024 Dec;15(1):2399247. doi: 10.1080/19491034.2024.2399247. Epub 2024 Sep 16. Nucleus. 2024. PMID: 39282864 Free PMC article. Review.
-
Cancer Therapeutic Proficiency of Dual-Targeted Mesoporous Silica Nanocomposite Endorses Combination Drug Delivery.ACS Omega. 2017 Nov 30;2(11):7959-7975. doi: 10.1021/acsomega.7b00978. Epub 2017 Nov 15. ACS Omega. 2017. PMID: 30023569 Free PMC article.
-
Barrier properties of Nup98 FG phases ruled by FG motif identity and inter-FG spacer length.Nat Commun. 2023 Feb 10;14(1):747. doi: 10.1038/s41467-023-36331-4. Nat Commun. 2023. PMID: 36765044 Free PMC article.
-
'Natively unfolded' nucleoporins in nucleocytoplasmic transport: clustered or evenly distributed?Nucleus. 2011 Jan-Feb;2(1):10-6. doi: 10.4161/nucl.2.1.13818. Nucleus. 2011. PMID: 21647294 Free PMC article.
References
-
- Bayliss, R., R.K. Ribbeck, D. Akin, H.M. Kent, C.M. Feldherr, D. Görlich, and M. Stewart. 1999. Interaction between NTF2 and xFxFG-containing nucleoporins is required to mediate nuclear import of RanGDP. J. Mol. Biol. 293:579–593. - PubMed
-
- Bodoor, K., S. Shaikh, D. Salina, W.H. Raharjo, R. Bastos, M. Lohka, and B. Burke. 1999. Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J. Cell Sci. 112:2253–2264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

