Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast
- PMID: 15657396
- PMCID: PMC2171581
- DOI: 10.1083/jcb.200408145
Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast
Abstract
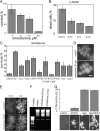
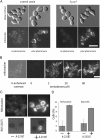
Although programmed cell death (PCD) is extensively studied in multicellular organisms, in recent years it has been shown that a unicellular organism, yeast Saccharomyces cerevisiae, also possesses death program(s). In particular, we have found that a high doses of yeast pheromone is a natural stimulus inducing PCD. Here, we show that the death cascades triggered by pheromone and by a drug amiodarone are very similar. We focused on the role of mitochondria during the pheromone/amiodarone-induced PCD. For the first time, a functional chain of the mitochondria-related events required for a particular case of yeast PCD has been revealed: an enhancement of mitochondrial respiration and of its energy coupling, a strong increase of mitochondrial membrane potential, both events triggered by the rise of cytoplasmic [Ca2+], a burst in generation of reactive oxygen species in center o of the respiratory chain complex III, mitochondrial thread-grain transition, and cytochrome c release from mitochondria. A novel mitochondrial protein required for thread-grain transition is identified.
Figures






References
-
- Ameisen, J.C. 1996. The origin of programmed cell death. Science. 272:1278–1279. - PubMed
-
- Bazhenova, E.N., Y.I. Deryabina, O. Eriksson, R.A. Zvyagilskaya, and N.E. Saris. 1998. Characterization of a high capacity calcium transport system in mitochondria of the yeast Endomyces magnusii. J. Biol. Chem. 273:4372–4377. - PubMed
-
- Breitenbach, M., F. Madeo, P. Laun, G. Heeren, S. Jarolim, K.-U. Fröhlich, S. Wissing, and A. Pichova. 2003. Yeast as a model for ageing and apoptosis research. Topics in Current Genetics. Vol. 3. Model Systems in Ageing. T. Nystrom and H.D. Osiewacz, editors. Springer-Verlag, Berlin/Heidelberg. 61–97.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

