ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation
- PMID: 15657398
- PMCID: PMC2171589
- DOI: 10.1083/jcb.200404008
ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation
Abstract
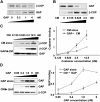
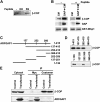
Examining how key components of coat protein I (COPI) transport participate in cargo sorting, we find that, instead of ADP ribosylation factor 1 (ARF1), its GTPase-activating protein (GAP) plays a direct role in promoting the binding of cargo proteins by coatomer (the core COPI complex). Activated ARF1 binds selectively to SNARE cargo proteins, with this binding likely to represent at least a mechanism by which activated ARF1 is stabilized on Golgi membrane to propagate its effector functions. We also find that the GAP catalytic activity plays a critical role in the formation of COPI vesicles from Golgi membrane, in contrast to the prevailing view that this activity antagonizes vesicle formation. Together, these findings indicate that GAP plays a central role in coupling cargo sorting and vesicle formation, with implications for simplifying models to describe how these two processes are coupled during COPI transport.
Figures


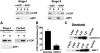

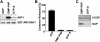
References
-
- Bigay, J., P. Gounon, S. Robineau, and B. Antonny. 2003. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature. 426:563–566. - PubMed
-
- Bonifacino, J.S., and B.S. Glick. 2004. The mechanisms of vesicle budding and fusion. Cell. 116:153–166. - PubMed
-
- Cosson, P., and F. Letourneur. 1994. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 263:1629–1631. - PubMed
-
- Cukierman, E., I. Huber, M. Rotman, and D. Cassel. 1995. The ARF1-GTPase-activating protein: zinc finger motif and Golgi complex localization. Science. 270:1999–2002. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

