A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis
- PMID: 15657400
- PMCID: PMC2171578
- DOI: 10.1083/jcb.200410091
A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis
Abstract
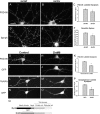
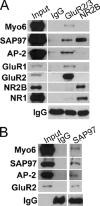
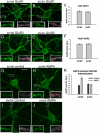
Myosin VI (Myo6) is an actin-based motor protein implicated in clathrin-mediated endocytosis in nonneuronal cells, though little is known about its function in the nervous system. Here, we find that Myo6 is highly expressed throughout the brain, localized to synapses, and enriched at the postsynaptic density. Myo6-deficient (Snell's waltzer; sv/sv) hippocampus exhibits a decrease in synapse number, abnormally short dendritic spines, and profound astrogliosis. Similarly, cultured sv/sv hippocampal neurons display decreased numbers of synapses and dendritic spines, and dominant-negative disruption of Myo6 in wild-type hippocampal neurons induces synapse loss. Importantly, we find that sv/sv hippocampal neurons display a significant deficit in the stimulation-induced internalization of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid-type glutamate receptors (AMPARs), and that Myo6 exists in a complex with the AMPAR, AP-2, and SAP97 in brain. These results suggest that Myo6 plays a role in the clathrin-mediated endocytosis of AMPARs, and that its loss leads to alterations in synaptic structure and astrogliosis.
Figures





References
-
- Avraham, K.B., T. Hasson, K.P. Steel, D.M. Kingsley, L.B. Russell, M.S. Mooseker, N.G. Copeland, and N.A. Jenkins. 1995. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat. Genet. 11:369–375. - PubMed
-
- Biemesderfer, D., S.A. Mentone, M. Mooseker, and T. Hasson. 2002. Expression of myosin VI within the early endocytic pathway in adult and developing proximal tubules. Am. J. Physiol. Renal Physiol. 282:F785–F794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

