Common effects of acidic activators on large-scale chromatin structure and transcription
- PMID: 15657424
- PMCID: PMC544008
- DOI: 10.1128/MCB.25.3.958-968.2005
Common effects of acidic activators on large-scale chromatin structure and transcription
Abstract
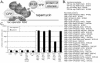
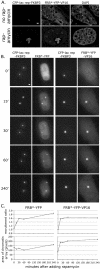
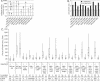
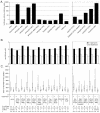
Large-scale chromatin decondensation has been observed after the targeting of certain acidic activators to heterochromatic chromatin domains. Acidic activators are often modular, with two or more separable transcriptional activation domains. Whether these smaller regions are sufficient for all functions of the activators has not been demonstrated. We adapted an inducible heterodimerization system to allow systematic dissection of the function of acidic activators, individual subdomains within these activators, and short acidic-hydrophobic peptide motifs within these subdomains. Here, we demonstrate that large-scale chromatin decondensation activity is a general property of acidic activators. Moreover, this activity maps to the same acidic activator subdomains and acidic-hydrophobic peptide motifs that are responsible for transcriptional activation. Two copies of a mutant peptide motif of VP16 (viral protein 16) possess large-scale chromatin decondensation activity but minimal transcriptional activity, and a synthetic acidic-hydrophobic peptide motif had large-scale chromatin decondensation activity comparable to the strongest full-length acidic activator but no transcriptional activity. Therefore, the general property of large-scale chromatin decondensation shared by most acidic activators is not simply a direct result of transcription per se but is most likely the result of the concerted action of coactivator proteins recruited by the activators' short acidic-hydrophobic peptide motifs.
Figures

Similar articles
-
Large-scale chromatin unfolding and remodeling induced by VP16 acidic activation domain.J Cell Biol. 1999 Jun 28;145(7):1341-54. doi: 10.1083/jcb.145.7.1341. J Cell Biol. 1999. PMID: 10385516 Free PMC article.
-
HMG17 is a chromatin-specific transcriptional coactivator that increases the efficiency of transcription initiation.Genes Dev. 1995 Aug 15;9(16):1978-91. doi: 10.1101/gad.9.16.1978. Genes Dev. 1995. PMID: 7649479
-
A minimal transcription activation domain consisting of a specific array of aspartic acid and leucine residues.Biol Chem Hoppe Seyler. 1994 Jul;375(7):463-70. doi: 10.1515/bchm3.1994.375.7.463. Biol Chem Hoppe Seyler. 1994. PMID: 7945996
-
[Viral transcription trans-activators].Biokhimiia. 1994 Feb;59(2):163-92. Biokhimiia. 1994. PMID: 8155778 Review. Russian.
-
Structure and functions of powerful transactivators: VP16, MyoD and FoxA.Int J Dev Biol. 2010;54(11-12):1589-96. doi: 10.1387/ijdb.103194hh. Int J Dev Biol. 2010. PMID: 21404180 Free PMC article. Review.
Cited by
-
Targeting chromosomal sites with locked nucleic acid-modified triplex-forming oligonucleotides: study of efficiency dependence on DNA nuclear environment.Nucleic Acids Res. 2006;34(16):4546-53. doi: 10.1093/nar/gkl630. Epub 2006 Sep 1. Nucleic Acids Res. 2006. PMID: 16951289 Free PMC article.
-
The SAGA chromatin-modifying complex: the sum of its parts is greater than the whole.Genes Dev. 2020 Oct 1;34(19-20):1287-1303. doi: 10.1101/gad.341156.120. Genes Dev. 2020. PMID: 33004486 Free PMC article. Review.
-
Epigenetic engineering: histone H3K9 acetylation is compatible with kinetochore structure and function.J Cell Sci. 2012 Jan 15;125(Pt 2):411-21. doi: 10.1242/jcs.090639. Epub 2012 Feb 13. J Cell Sci. 2012. PMID: 22331359 Free PMC article.
-
Cell cycle-dependent regulation of Saccharomyces cerevisiae donor preference during mating-type switching by SBF (Swi4/Swi6) and Fkh1.Mol Cell Biol. 2006 Jul;26(14):5470-80. doi: 10.1128/MCB.02443-05. Mol Cell Biol. 2006. PMID: 16809780 Free PMC article.
-
Structural insights into p300 regulation and acetylation-dependent genome organisation.Nat Commun. 2022 Dec 15;13(1):7759. doi: 10.1038/s41467-022-35375-2. Nat Commun. 2022. PMID: 36522330 Free PMC article.
References
-
- Belmont, A. S. 2001. Visualizing chromosome dynamics with GFP. Trends Cell Biol. 11:250-257. - PubMed
-
- Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. - PubMed
-
- Berger, S. L., W. D. Cress, A. Cress, S. J. Triezenberg, and L. Guarente. 1990. Selective inhibition of activated but not basal transcription by the acidic activation domain of VP16: evidence for transcriptional adaptors. Cell 61:1199-1208. - PubMed
-
- Carpenter, A. E., A. Ashouri, and A. S. Belmont. 2004. Automated microscopy identifies estrogen receptor subdomains with large-scale chromatin structure unfolding activity. Cytometry 58A:157-166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
