Regulation of Rho and Rac signaling to the actin cytoskeleton by paxillin during Drosophila development
- PMID: 15657426
- PMCID: PMC544021
- DOI: 10.1128/MCB.25.3.979-987.2005
Regulation of Rho and Rac signaling to the actin cytoskeleton by paxillin during Drosophila development
Abstract
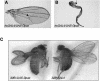
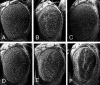
Paxillin is a prominent focal adhesion docking protein that regulates cell adhesion and migration. Although numerous paxillin-binding proteins have been identified and paxillin is required for normal embryogenesis, the precise mechanism by which paxillin functions in vivo has not yet been determined. We identified an ortholog of mammalian paxillin in Drosophila (Dpax) and have undertaken a genetic analysis of paxillin function during development. Overexpression of Dpax disrupted leg and wing development, suggesting a role for paxillin in imaginal disc morphogenesis. These defects may reflect a function for paxillin in regulation of Rho family GTPase signaling as paxillin interacts genetically with Rac and Rho in the developing eye. Moreover, a gain-of-function suppressor screen identified a genetic interaction between Dpax and cdi in wing development. cdi belongs to the cofilin kinase family, which includes the downstream Rho target, LIM kinase (LIMK). Significantly, strong genetic interactions were detected between Dpax and Dlimk, as well as downstream effectors of Dlimk. Supporting these genetic data, biochemical studies indicate that paxillin regulates Rac and Rho activity, positively regulating Rac and negatively regulating Rho. Taken together, these data indicate the importance of paxillin modulation of Rho family GTPases during development and identify the LIMK pathway as a critical target of paxillin-mediated Rho regulation.
Figures




References
-
- Bagrodia, S., S. J. Taylor, K. A. Jordon, L. Van Aelst, and R. A. Cerione. 1998. A novel regulator of p21-activated kinases J. Biol. Chem. 273:23633-23636. - PubMed
-
- Brabant, M. C., and D. L. Brower. 1993. PS2 integrin requirements in Drosophila embryo and wing morphogenesis. Dev. Biol. 157:49-59. - PubMed
-
- Brown, M. C., J. A. Perrotta, C. E. Turner, and J. T. Miller. 1996. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J. Cell Biol. 135:1109-1123. - PMC - PubMed
-
- Burridge, K. 1999. Crosstalk between Rac and Rho. Science 283:2028-2029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
