Human papillomavirus oncoprotein E7 targets the promyelocytic leukemia protein and circumvents cellular senescence via the Rb and p53 tumor suppressor pathways
- PMID: 15657429
- PMCID: PMC543993
- DOI: 10.1128/MCB.25.3.1013-1024.2005
Human papillomavirus oncoprotein E7 targets the promyelocytic leukemia protein and circumvents cellular senescence via the Rb and p53 tumor suppressor pathways
Retraction in
-
Retraction for Bischof et al., "Human Papillomavirus Oncoprotein E7 Targets the Promyelocytic Leukemia Protein and Circumvents Cellular Senescence via the Rb and p53 Tumor Suppressor Pathways".Mol Cell Biol. 2020 Oct 26;40(22):e00482-20. doi: 10.1128/MCB.00482-20. Print 2020 Oct 26. Mol Cell Biol. 2020. PMID: 33106371 Free PMC article. No abstract available.
Abstract
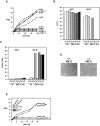
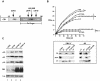
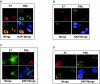
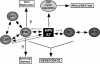
Cellular senescence can be triggered by a variety of signals, including loss of telomeric integrity or intense oncogenic signaling, and is considered a potent, natural tumor suppressor mechanism. Previously, it was shown that the promyelocytic leukemia protein (PML) induces cellular senescence when overexpressed in primary human fibroblasts. The mechanism by which the PML IV isoform elicits this irreversible growth arrest is believed to involve activation of the tumor suppressor pathways p21/p53 and p16/Rb; however, a requirement for either pathway has not been demonstrated unequivocally. To investigate the individual contributions of p53 and Rb to PML-induced senescence, we used oncoproteins E6 and E7 from human papillomaviruses (HPVs), which predominantly target p53 and Rb. We show that E7, but not E6, circumvents PML-induced senescence. Using different E7 mutant proteins, dominant negative cyclin-dependent kinase 4, and p16 RNA interference, we demonstrate that Rb-related and Rb-independent mechanisms of E7 are necessary for subversion of PML-induced senescence and we identify PML as a novel target for E7. Interaction between E7 and a functional prosenescence complex composed of PML, p53, and CBP perturbs transcriptional activation of p53, thus highlighting a significant effect also on the p53 tumor suppressor pathway. Given the importance of HPV in the pathogenesis of cervical cancer, our results warrant a more detailed analyses of PML in HPV infections.
Figures


References
-
- Appella, E., and C. W. Anderson. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268:2764-2772. - PubMed
-
- Avvakumov, N., J. Torchia, and J. S. Mymryk. 2003. Interaction of the HPV E7 proteins with the pCAF acetyltransferase. Oncogene 22:3833-3841. - PubMed
-
- Bartkova, J., J. Lukas, H. Muller, M. Strauss, B. Gusterson, and J. Bartek. 1995. Abnormal patterns of D-type cyclin expression and G1 regulation in human head and neck cancer. Cancer Res. 55:949-956. - PubMed
-
- Bernat, A., N. Avvakumov, J. S. Mymryk, and L. Banks. 2003. Interaction between the HPV E7 oncoprotein and the transcriptional coactivator p300. Oncogene 22:7871-7881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
