Combination treatment of CC531-lac-Z rat liver metastases by chemoembolization with pemetrexed disodium and gemcitabine
- PMID: 15657768
- PMCID: PMC12161237
- DOI: 10.1007/s00432-004-0643-y
Combination treatment of CC531-lac-Z rat liver metastases by chemoembolization with pemetrexed disodium and gemcitabine
Abstract
Purpose: The aim of this study was to evaluate the combination effect of pemetrexed disodium (MTA; Alimta; LY 231514) and gemcitabine (GEM) administered by hepatic artery and portal vein chemoembolization (HACE and PVCE) in a colorectal cancer rat liver metastasis model.
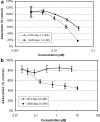
Materials and methods: Proliferation studies on CC531-lac-Z rat colon cancer cells were performed using the MTT assay to obtain the optimal combination schedule of the two antineoplastic agents. To generate diffuse liver metastasis, 4 x 10(6) tumor cells were implanted into the portal vein of male WAG/Rij rats. MTA (30 mg/kg, 60 mg/kg, and 90 mg/kg) was administered locoregionally by portal vein chemoembolization (PVCE) and compared with repeated systemic intravenous injection. GEM (50 mg/kg) was also given locoregionally by hepatic artery chemoembolization (HACE) as well as systemically. All routes of administration were examined alone as well as in combination. Efficacy of treatment in terms of liver metastases burden was determined at the end of the experiment by measuring the beta-galactosidase activity of CC531-lac-Z cells with a chemoluminescence assay.
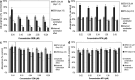
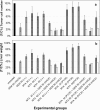
Results: Combination experiments in vitro showed a more than additive tumor cell reduction after sequential exposure to MTA preceding GEM (observed/expected ratio [O/E] = 0.73). Experiments with the reverse sequence (GEM-->MTA) resulted only in additive combination effects (O/E ratio = 1.08). Simultaneous drug exposure showed less than additive combination effects (O/E ratios > or = 1.25). In vivo, locoregional administration by HACE with GEM was significantly more effective than systemic intravenous bolus treatment (P = 0.03). Portal vein chemoembolization with MTA performed immediately after tumor cell inoculation was ineffective. Repeated systemic treatment with MTA yielded a slight reduction in tumor cell load that was significant versus control at the medium and high doses (60 mg/kg, P = 0.009; 90 mg/kg, P = 0.046) but not versus intraportal chemoembolization. The combination treatment of systemic (60 and 90 mg/kg) or locoregional (60 mg/kg) MTA with HACE using GEM (50 mg/kg) resulted in more than 80% tumor growth inhibition; this antineoplastic combination effect was maximally additive.
Conclusion: A regimen-dependent synergistic combination effect of both drugs was found in vitro. In animals, hepatic artery chemoembolization with GEM was superior to systemic intravenous bolus treatment. Portal vein chemoembolization with MTA was ineffective. The optimal in vitro regimen of MTA (intravenous or PVCE) preceding GEM (HACE) resulted in a maximally additive tumor growth inhibition. The results indicate that MTA and GEM can successfully be combined and favor further evaluation in patients.
Figures


References
-
- Ackerman NB (1974) The blood supply of experimental liver metastases. IV. Changes in vascularity with increasing tumor growth. Surgery. 75:589–596 - PubMed
-
- Adam R (2003) Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol 14(suppl 2):II13–II16 - PubMed
-
- Adjei AA (2001) Gemcitabine and pemetrexed disodium combinations in vitro and in vivo. Lung Cancer 34(suppl 4):S103–S105 - PubMed
-
- Adjei AA (2002) Pemetrexed in the treatment of selected solid tumors. Semin Oncol 29:50–53 - PubMed
-
- Adjei AA (2004) Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent. Clin Cancer Res 10:4276s–4280s - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

