Stability of HAMLET--a kinetically trapped alpha-lactalbumin oleic acid complex
- PMID: 15659367
- PMCID: PMC2253409
- DOI: 10.1110/ps.04982905
Stability of HAMLET--a kinetically trapped alpha-lactalbumin oleic acid complex
Abstract
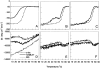
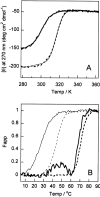
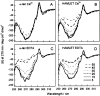
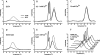
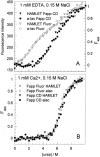
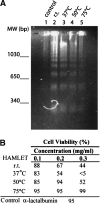
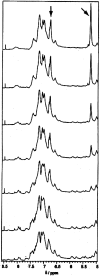
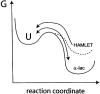
The stability toward thermal and urea denaturation was measured for HAMLET (human alpha-lactalbumin made lethal to tumor cells) and alpha-lactalbumin, using circular dichroism and fluorescence spectroscopy as well as differential scanning calorimetry. Under all conditions examined, HAMLET appears to have the same or lower stability than alpha-lactalbumin. The largest difference is seen for thermal denaturation of the calcium free (apo) forms, where the temperature at the transition midpoint is 15 degrees C lower for apo HAMLET than for apo alpha-lactalbumin. The difference becomes progressively smaller as the calcium concentration increases. Denaturation of HAMLET was found to be irreversible. Samples of HAMLET that have been renatured after denaturation have lost the specific biological activity toward tumor cells. Three lines of evidence indicate that HAMLET is a kinetic trap: (1) It has lower stability than alpha-lactalbumin, although it is a complex of alpha-lactalbumin and oleic acid; (2) its denaturation is irreversible and HAMLET is lost after denaturation; (3) formation of HAMLET requires a specific conversion protocol.
Figures

References
-
- Acharya, K.R., Stuart, D.I., Walker, N.P., Lewis, M., and Phillips, D.C. 1989. Refined structure of baboon α-lactalbumin at 1.7 Å resolution. Comparison with c-type lysozyme. J. Mol. Biol. 208 99–127. - PubMed
-
- Acharya, K.R., Ren, J.S., Stuart, D.I., Phillips, D.C., and Fenna, R.E. 1991. Crystal structure of human α-lactalbumin at 1.7 Å resolution. J. Mol. Biol. 221 571–581. - PubMed
-
- Baker, D. and Agard, D.A. 1994. Kinetics versus thermodynamics in protein folding. Biochemistry 33 7505–7509. - PubMed
-
- Baldwin, T.O., Ziegler, M.M., Chaffotte, A.F., and Goldberg, M.E. 1993. Contribution of folding steps involving the individual subunits of bacterial luciferase to the assembly of the active heterodimeric enzyme. J. Biol. Chem. 268 10766–10772. - PubMed
-
- Brew, K. and Hill, R.L. 1975. Lactose biosynthesis. Rev. Physiol. Biochem. Pharmacol. 72 105–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

