Folding and particle assembly are disrupted by single-point mutations near the autocatalytic cleavage site of Nudaurelia capensis omega virus capsid protein
- PMID: 15659373
- PMCID: PMC2253427
- DOI: 10.1110/ps.041054605
Folding and particle assembly are disrupted by single-point mutations near the autocatalytic cleavage site of Nudaurelia capensis omega virus capsid protein
Abstract
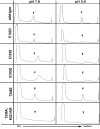
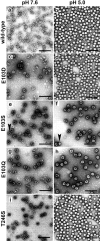
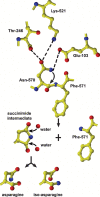
Protein subunits of several RNA viruses are known to undergo post-assembly, autocatalytic cleavage that is required for infectivity. Nudaurelia capensis omega virus (Nomega V) is one of the simplest viruses to undergo an autocatalytic cleavage, making it an excellent model to understand both assembly and the mechanism of autoproteolysis. Heterologous expression of the coat protein gene of Nomega V in a baculovirus system results in the spontaneous assembly of virus-like particles (VLPs) that remain uncleaved when purified at neutral pH. After acidification to pH 5.0, the VLPs autocatalytically cleave at residue 570, providing an in vitro control of the cleavage. The crystal structure of Nomega V displays three residues near the scissile bond that were candidates for participation in the reaction. These were changed by site-directed mutagenesis to conservative and nonconservative residues and the products analyzed. Even conservative changes at the three residues dramatically reduced cleavage when the subunits assembled properly. Unexpectedly, we discovered that these residues are not only critical to the kinetics of Nomega V autoproteolysis, but are also necessary for proper folding of subunits and, ultimately, assembly of Nomega V VLPs.
Figures


Similar articles
-
The refined structure of Nudaurelia capensis omega virus reveals control elements for a T = 4 capsid maturation.Virology. 2004 Jan 5;318(1):192-203. doi: 10.1016/j.virol.2003.08.045. Virology. 2004. PMID: 14972547
-
Characterization of large conformational changes and autoproteolysis in the maturation of a T=4 virus capsid.J Virol. 2009 Jan;83(2):1126-34. doi: 10.1128/JVI.01859-08. Epub 2008 Nov 5. J Virol. 2009. PMID: 18987141 Free PMC article.
-
Large conformational changes in the maturation of a simple RNA virus, nudaurelia capensis omega virus (NomegaV).J Mol Biol. 2000 Jun 9;299(3):573-84. doi: 10.1006/jmbi.2000.3723. J Mol Biol. 2000. PMID: 10835268
-
Dynamics and stability in the maturation of a eukaryotic virus: a paradigm for chemically programmed large-scale macromolecular reorganization.Arch Virol. 2021 Jun;166(6):1547-1563. doi: 10.1007/s00705-021-05007-z. Epub 2021 Mar 8. Arch Virol. 2021. PMID: 33683475 Review.
-
Assembly and maturation of a T = 4 quasi-equivalent virus is guided by electrostatic and mechanical forces.Viruses. 2014 Aug 22;6(8):3348-62. doi: 10.3390/v6083348. Viruses. 2014. PMID: 25153346 Free PMC article. Review.
Cited by
-
Essential autoproteolysis of bacterial anti-σ factor RsgI for transmembrane signal transduction.Sci Adv. 2023 Jul 7;9(27):eadg4846. doi: 10.1126/sciadv.adg4846. Epub 2023 Jul 7. Sci Adv. 2023. PMID: 37418529 Free PMC article.
-
Subunits fold at position-dependent rates during maturation of a eukaryotic RNA virus.Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14111-5. doi: 10.1073/pnas.1004221107. Epub 2010 Jul 26. Proc Natl Acad Sci U S A. 2010. PMID: 20660783 Free PMC article.
-
Autoproteolytic mechanism of CdiA toxin release reconstituted in vitro.J Bacteriol. 2024 Oct 24;206(10):e0024924. doi: 10.1128/jb.00249-24. Epub 2024 Sep 30. J Bacteriol. 2024. PMID: 39347575 Free PMC article.
-
Structures of honeybee-infecting Lake Sinai virus reveal domain functions and capsid assembly with dynamic motions.Nat Commun. 2023 Feb 1;14(1):545. doi: 10.1038/s41467-023-36235-3. Nat Commun. 2023. PMID: 36726015 Free PMC article.
-
Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism.EMBO J. 2007 Apr 4;26(7):1942-52. doi: 10.1038/sj.emboj.7601638. Epub 2007 Mar 8. EMBO J. 2007. PMID: 17347646 Free PMC article.
References
-
- Agrawal, D. and Johnson, J. 1992. Sequence and analysis of the capsid protein of Nudaurelia capensis ω Virus, an insect virus with T = 4 icosahedral symmetry. Virology 190 806–814. - PubMed
-
- ———. 1995. Assembly of the T = 4 Nudaurelia capensis ω virus capsid protein, post translational cleavage, and specific encapsidation of its mRNA in a baculovirus expression system. Virology 207 89–97. - PubMed
-
- Borsa, J., Morash, B.D., Sargent, M.D., Copps, T.P., Lievaart, P.A., and Szekely, J.G. 1979. Two modes of entry of reovirus particles into L cells. J. Gen. Virol. 45 161–170. - PubMed
-
- Briehl, R.W. 1980. Solid-like behaviour of unsheared sickle haemoglobin gels and the effects of shear. Nature 288 622–624. - PubMed
-
- Canady, M.A., Tihova, M., Hanzlik, T.N., Johnson, J.E., and Yeager, M. 2000. Large conformational changes in the maturation of a simple RNA virus, Nudaurelia capensis ω virus (NωV). J. Mol. Biol. 299 573–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

