Specific erythrocyte binding capacity and biological activity of Plasmodium falciparum erythrocyte binding ligand 1 (EBL-1)-derived peptides
- PMID: 15659376
- PMCID: PMC2254251
- DOI: 10.1110/ps.041084305
Specific erythrocyte binding capacity and biological activity of Plasmodium falciparum erythrocyte binding ligand 1 (EBL-1)-derived peptides
Abstract
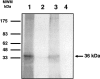
Erythrocyte binding ligand 1 (EBL-1) is a member of the ebl multigene family involved in Plasmodium falciparum invasion of erythrocytes. We found that five EBL-1 high-activity binding peptides (HABPs) bound specifically to erythrocytes: 29895 ((41)HKKKSGELNNNKSGILRSTY(60)), 29903 ((201)LYECGK-KIKEMKWICTDNQF(220)), 29923 ((601)CNAILGSYADIGDIVRGLDV(620)), 29924((621)WRDINTNKLSEK-FQKIFMGGY(640)), and 30018 ((2481)LEDIINLSKKKKKSINDTSFY(2500)). We also show that binding was saturable, not sialic acid-dependent, and that all peptides specifically bound to a 36-kDa protein on the erythrocyte membrane. The five HABPs inhibited in vitro merozoite invasion depending on the peptide concentration used, suggesting their possible role in the invasion process.
Figures




Similar articles
-
Plasmodium falciparum merozoite surface protein 6 (MSP-6) derived peptides bind erythrocytes and partially inhibit parasite invasion.Peptides. 2006 Jul;27(7):1685-92. doi: 10.1016/j.peptides.2006.02.001. Epub 2006 May 19. Peptides. 2006. PMID: 16713025
-
Identifying Plasmodium falciparum merozoite surface antigen 3 (MSP3) protein peptides that bind specifically to erythrocytes and inhibit merozoite invasion.Protein Sci. 2005 Jul;14(7):1778-86. doi: 10.1110/ps.041304505. Protein Sci. 2005. PMID: 15987906 Free PMC article.
-
Specific erythrocyte binding capacity and biological activity of Plasmodium falciparum-derived rhoptry-associated protein 1 peptides.Vaccine. 2004 Feb 25;22(8):1054-62. doi: 10.1016/j.vaccine.2003.07.019. Vaccine. 2004. PMID: 15161083
-
Disguising itself--insights into Plasmodium falciparum binding and immune evasion from the DBL crystal structure.Mol Biochem Parasitol. 2006 Jul;148(1):1-9. doi: 10.1016/j.molbiopara.2006.03.004. Epub 2006 Apr 4. Mol Biochem Parasitol. 2006. PMID: 16621067 Review.
-
[Human erythrocyte glycophorin C as the receptor for EBA-140 Plasmodium falciparum merozoite ligand].Postepy Hig Med Dosw (Online). 2013 Dec 23;67:1331-9. doi: 10.5604/17322693.1081865. Postepy Hig Med Dosw (Online). 2013. PMID: 24379273 Review. Polish.
Cited by
-
Erythrocyte glycophorins as receptors for Plasmodium merozoites.Parasit Vectors. 2019 Jun 24;12(1):317. doi: 10.1186/s13071-019-3575-8. Parasit Vectors. 2019. PMID: 31234897 Free PMC article. Review.
-
Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1.Proc Natl Acad Sci U S A. 2009 Mar 31;106(13):5348-52. doi: 10.1073/pnas.0900878106. Epub 2009 Mar 11. Proc Natl Acad Sci U S A. 2009. PMID: 19279206 Free PMC article.
-
Identification of a specific region of Plasmodium falciparum EBL-1 that binds to host receptor glycophorin B and inhibits merozoite invasion in human red blood cells.Mol Biochem Parasitol. 2012 May;183(1):23-31. doi: 10.1016/j.molbiopara.2012.01.002. Epub 2012 Jan 16. Mol Biochem Parasitol. 2012. PMID: 22273481 Free PMC article.
-
Mycobacterium tuberculosis Rv0679c protein sequences involved in host-cell infection: potential TB vaccine candidate antigen.BMC Microbiol. 2010 Apr 13;10:109. doi: 10.1186/1471-2180-10-109. BMC Microbiol. 2010. PMID: 20388213 Free PMC article.
-
The Cellular and Molecular Interaction Between Erythrocytes and Plasmodium falciparum Merozoites.Front Cell Infect Microbiol. 2022 Mar 31;12:816574. doi: 10.3389/fcimb.2022.816574. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35433504 Free PMC article. Review.
References
-
- Adams, J.H., Blair, P.L., Kaneko, O., and Peterson, D.S. 2001. An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 17 297–299. - PubMed
-
- Camus, D. and Hadley, T.J. 1985. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230 553–556. - PubMed
-
- Carvalho, L.J., Daniel-Ribeiro, C.T., and Goto, H. 2002. Malaria vaccine: Candidate antigens, mechanisms, constraints and prospects. Scand. J. Immunol. 56 327–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

