The trans-Golgi network-associated human ubiquitin-protein ligase POSH is essential for HIV type 1 production
- PMID: 15659549
- PMCID: PMC545085
- DOI: 10.1073/pnas.0408717102
The trans-Golgi network-associated human ubiquitin-protein ligase POSH is essential for HIV type 1 production
Abstract
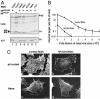
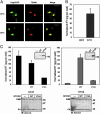
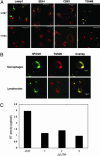
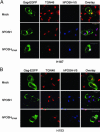
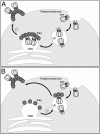
HIV type 1 (HIV-1) was shown to assemble either at the plasma membrane or in the membrane of late endosomes. Now, we report an essential role for human ubiquitin ligase POSH (Plenty of SH3s; hPOSH), a trans-Golgi network-associated protein, in the targeting of HIV-1 to the plasma membrane. Small inhibitory RNA-mediated silencing of hPOSH ablates virus secretion and Gag plasma membrane localization. Reintroduction of native, but not a RING finger mutant, hPOSH restores virus release and Gag plasma membrane localization in hPOSH-depleted cells. Furthermore, expression of the RING finger mutant hPOSH inhibits virus release and induces accumulation of intracellular Gag in normal cells. Together, our results identify a previously undescribed step in HIV biogenesis and suggest a direct function for hPOSH-mediated ubiquitination in protein sorting at the trans-Golgi network. Consequently, hPOSH may be a useful host target for therapeutic intervention.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

