Synaptic targeting of retrogradely transported trophic factors in motoneurons: comparison of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, and cardiotrophin-1 with tetanus toxin
- PMID: 15659589
- PMCID: PMC6725325
- DOI: 10.1523/JNEUROSCI.4322-04.2005
Synaptic targeting of retrogradely transported trophic factors in motoneurons: comparison of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, and cardiotrophin-1 with tetanus toxin
Abstract
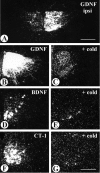
Glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and cardiotrophin-1 (CT-1) are the most potent neurotrophic factors for motoneurons, but their fate after retrograde axonal transport is not known. Internalized trophic factors may be degraded, or they may be recycled and transferred to other neurons, similar to the known route of tetanus toxin. We tested whether neonatal rat hypoglossal motoneurons target retrogradely transported trophic factors to synaptic sites on their dendrites within the brainstem and subsequently transfer these trophins across the synaptic cleft to afferent synapses (transsynaptic transcytosis). Motoneurons retrogradely transport from the tongue radiolabeled GDNF, BDNF, and CT-1 as well as tetanus toxin. Quantitative autoradiographic electron microscopy showed that GDNF and BDNF were transported into motoneuron dendrites with labeling densities similar to those of tetanus toxin. Although tetanus toxin accumulated rapidly (within 8 h) at presynaptic sites, GDNF accumulated at synapses more slowly (within 15 h), and CT-1 never associated with synapses. Thus, some retrogradely transported neurotrophic factors are trafficked similarly but not identically to tetanus toxin. Both GDNF and BDNF accumulate at the external (limiting) membrane of multivesicular bodies within proximal dendrites. We conclude that tetanus toxin, GDNF, and BDNF are released from postsynaptic sites and are internalized by afferent presynaptic terminals, thus demonstrating transsynaptic transcytosis. CT-1, however, follows a strict degradation pathway after retrograde transport to the soma. Synaptic and transcytotic trafficking thus are restricted to particular neurotrophic factors such as GDNF and BDNF.
Figures





Similar articles
-
Selective retrograde transsynaptic transfer of a protein, tetanus toxin, subsequent to its retrograde axonal transport.J Cell Biol. 1979 Sep;82(3):798-810. doi: 10.1083/jcb.82.3.798. J Cell Biol. 1979. PMID: 92475 Free PMC article.
-
Quantitative analysis of multivesicular bodies (MVBs) in the hypoglossal nerve: evidence that neurotrophic factors do not use MVBs for retrograde axonal transport.J Comp Neurol. 2009 Jun 20;514(6):641-57. doi: 10.1002/cne.22047. J Comp Neurol. 2009. PMID: 19363811 Free PMC article.
-
Transsynaptic transfer of retrogradely transported tetanus protein-peroxidase conjugates.Exp Neurol. 1989 Nov;106(2):197-203. doi: 10.1016/0014-4886(89)90094-0. Exp Neurol. 1989. PMID: 2478384
-
[Axonal transport from the nerve ending to the nerve cell body: a pathway for trophic signals and neurotoxins].Bull Schweiz Akad Med Wiss. 1980 Apr;36(1-3):7-19. Bull Schweiz Akad Med Wiss. 1980. PMID: 6159028 Review. German.
-
Anterograde axonal transport, transcytosis, and recycling of neurotrophic factors: the concept of trophic currencies in neural networks.Mol Neurobiol. 2001 Aug-Dec;24(1-3):1-28. doi: 10.1385/MN:24:1-3:001. Mol Neurobiol. 2001. PMID: 11831547 Review.
Cited by
-
Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina.Mol Vis. 2012;18:920-36. Epub 2012 Apr 12. Mol Vis. 2012. PMID: 22539871 Free PMC article.
-
Botulinum Neurotoxin Application to the Severed Femoral Nerve Modulates Spinal Synaptic Responses to Axotomy and Enhances Motor Recovery in Rats.Neural Plast. 2018 Sep 5;2018:7975013. doi: 10.1155/2018/7975013. eCollection 2018. Neural Plast. 2018. PMID: 30254669 Free PMC article.
-
Size, Shape, and Distribution of Multivesicular Bodies in the Juvenile Rat Somatosensory Cortex: A 3D Electron Microscopy Study.Cereb Cortex. 2020 Mar 14;30(3):1887-1901. doi: 10.1093/cercor/bhz211. Cereb Cortex. 2020. PMID: 31665237 Free PMC article.
-
Fragment C of tetanus toxin, more than a carrier. Novel perspectives in non-viral ALS gene therapy.J Mol Med (Berl). 2010 Mar;88(3):297-308. doi: 10.1007/s00109-009-0556-y. J Mol Med (Berl). 2010. PMID: 19921501
-
Information handling by the brain: proposal of a new "paradigm" involving the roamer type of volume transmission and the tunneling nanotube type of wiring transmission.J Neural Transm (Vienna). 2014 Dec;121(12):1431-49. doi: 10.1007/s00702-014-1240-0. Epub 2014 May 28. J Neural Transm (Vienna). 2014. PMID: 24866694 Review.
References
-
- Blesch A, Tuszynski MH (2001) GDNF gene delivery to injured adult CNS motor neurons promotes axonal growth, expression of the trophic neuropeptide CGRP, and cellular protection. J Comp Neurol 436: 399-410. - PubMed
-
- Bordet T, Castelnau-Ptakhine L, Fauchereau F, Friocourt G, Kahn A, Haase G (2001) Neuronal targeting of cardiotrophin-1 by coupling with tetanus toxin C fragment. Mol Cell Neurosci 17: 842-854. - PubMed
-
- Bourque MJ, Trudeau LE (2000) GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci 12: 3172-3180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
