Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum
- PMID: 15659593
- PMCID: PMC6725320
- DOI: 10.1523/JNEUROSCI.4196-04.2005
Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum
Abstract
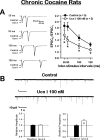
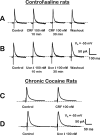
Corticotropin-releasing factor (CRF) and urocortin (Ucn I) are endogenous members among a family of CRF-related peptides that activate two different and synaptically localized G-protein-coupled receptors, CRF1 and CRF2. These peptides and their receptors have been implicated in stress responses and stress with cocaine abuse. In this study, we observed significant alterations in excitatory transmission and CRF-related peptide regulation of excitatory transmission in the lateral septum mediolateral nucleus (LSMLN) after chronic cocaine administration. In brain slice recordings from the LSMLN of control (saline-treated) rats, glutamatergic synaptic transmission was facilitated by activation of CRF1 receptors with CRF but was depressed after activation of CRF2 receptors with Ucn I. After acute withdrawal from a chronic cocaine administration regimen, CRF1 activation remained facilitatory, but CRF2 activation facilitated rather than depressed LSMLN EPSCs. These alterations in CRF2 effects occurred through both presynaptic and postsynaptic mechanisms. In saline-treated rats, CRF1 and CRF2 coupled predominantly to protein kinase A signaling pathways, whereas after cocaine withdrawal, protein kinase C activity was more prominent and likely contributed to the CRF2-mediated presynaptic facilitation. Neither CRF nor Ucn I altered monosynaptic GABA(A)-mediated IPSCs before or after chronic cocaine administration, suggesting that loss of GABAA-mediated inhibition could not account for the facilitation. This switch in polarity of Ucn I-mediated neuromodulation, from a negative to positive regulation of excitatory glutamatergic transmission after chronic cocaine administration, could generate an imbalance in the brain reward circuitry associated with the LSMLN.
Figures
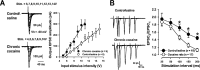


References
-
- Dunn AJ, Berridge CW (1990) Is corticotropin-releasing factor a mediator of stress responses? Ann NY Acad Sci 579: 183-191. - PubMed
-
- Erb S, Shaham Y, Stewart J (1996) Stress re-instates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology 128: 408-412. - PubMed
-
- Franklin TR, Druhan JP (2000) Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci 12: 2097-2106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
