Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin
- PMID: 15659595
- PMCID: PMC2275318
- DOI: 10.1523/JNEUROSCI.4011-04.2005
Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin
Abstract
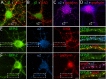
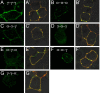
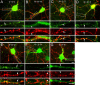
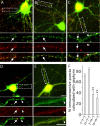
Modulation of the concentration of postsynaptic GABA(A) receptors contributes to functional plasticity of inhibitory synapses. The gamma2 subunit of GABA(A) receptor is specifically required for clustering of these receptors, for recruitment of the submembrane scaffold protein gephyrin to postsynaptic sites, and for postsynaptic function of GABAergic inhibitory synapses. To elucidate this mechanism, we here have mapped the gamma2 subunit domains required for restoration of postsynaptic clustering and function of GABA(A) receptors in gamma2 subunit mutant neurons. Transfection of gamma2-/- neurons with the gamma2 subunit but not the alpha2 subunit rescues postsynaptic clustering of GABA(A) receptors, results in recruitment of gephyrin to postsynaptic sites, and restores the amplitude and frequency of miniature inhibitory postsynaptic currents to wild-type levels. Analogous analyses of chimeric gamma2/alpha2 subunit constructs indicate, unexpectedly, that the fourth transmembrane domain of the gamma2 subunit is required and sufficient for postsynaptic clustering of GABA(A) receptors, whereas cytoplasmic gamma2 subunit domains are dispensable. In contrast, both the major cytoplasmic loop and the fourth transmembrane domain of the gamma2 subunit contribute to efficient recruitment of gephyrin to postsynaptic receptor clusters and are essential for restoration of miniature IPSCs. Our study points to a novel mechanism involved in targeting of GABA(A) receptors and gephyrin to inhibitory synapses.
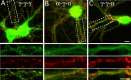
Figures





References
-
- Banker G, Goslin K (1998) Culturing nerve cells. Cambridge, MA: MIT.
-
- Benson JA, Low K, Keist R, Mohler H, Rudolph U (1998) Pharmacology of recombinant γ-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated α-subunits. FEBS Lett 431: 400-404. - PubMed
-
- Blanton MP, Cohen JB (1994) Identifying the lipid-protein interface of the Torpedo nicotinic acetylcholine receptor: secondary structure implications. Biochemistry 33: 2859-2872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
