Ensemble coding of vocal control in birdsong
- PMID: 15659602
- PMCID: PMC6725314
- DOI: 10.1523/JNEUROSCI.3036-04.2005
Ensemble coding of vocal control in birdsong
Abstract
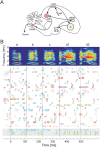
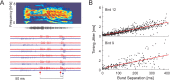
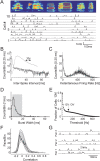
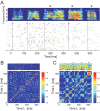
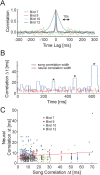
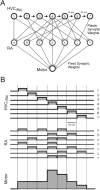
Zebra finch song is represented in the high-level motor control nucleus high vocal center (HVC) (Reiner et al., 2004) as a sparse sequence of spike bursts. In contrast, the vocal organ is driven continuously by smoothly varying muscle control signals. To investigate how the sparse HVC code is transformed into continuous vocal patterns, we recorded in the singing zebra finch from populations of neurons in the robust nucleus of arcopallium (RA), a premotor area intermediate between HVC and the motor neurons. We found that highly similar song elements are typically produced by different RA ensembles. Furthermore, although the song is modulated on a wide range of time scales (10-100 ms), patterns of neural activity in RA change only on a short time scale (5-10 ms). We suggest that song is driven by a dynamic circuit that operates on a single underlying clock, and that the large convergence of RA neurons to vocal control muscles results in a many-to-one mapping of RA activity to song structure. This permits rapidly changing RA ensembles to drive both fast and slow acoustic modulations, thereby transforming the sparse HVC code into a continuous vocal pattern.
Figures


Similar articles
-
Singing-related activity of identified HVC neurons in the zebra finch.J Neurophysiol. 2007 Jun;97(6):4271-83. doi: 10.1152/jn.00952.2006. Epub 2006 Dec 20. J Neurophysiol. 2007. PMID: 17182906
-
A distributed neural network model for the distinct roles of medial and lateral HVC in zebra finch song production.J Neurophysiol. 2017 Aug 1;118(2):677-692. doi: 10.1152/jn.00917.2016. Epub 2017 Apr 5. J Neurophysiol. 2017. PMID: 28381490 Free PMC article.
-
Acute inhibition of a cortical motor area impairs vocal control in singing zebra finches.Eur J Neurosci. 2015 Jan;41(1):97-108. doi: 10.1111/ejn.12757. Epub 2014 Oct 29. Eur J Neurosci. 2015. PMID: 25354166
-
Two neural streams, one voice: pathways for theme and variation in the songbird brain.Neuroscience. 2014 Sep 26;277:806-17. doi: 10.1016/j.neuroscience.2014.07.061. Epub 2014 Aug 8. Neuroscience. 2014. PMID: 25106128 Review.
-
A species-specific view of song representation in a sensorimotor nucleus.J Physiol Paris. 2013 Jun;107(3):193-202. doi: 10.1016/j.jphysparis.2012.08.004. Epub 2012 Aug 30. J Physiol Paris. 2013. PMID: 22960663 Review.
Cited by
-
HTR2 receptors in a songbird premotor cortical-like area modulate spectral characteristics of zebra finch song.J Neurosci. 2013 Feb 13;33(7):2908-15. doi: 10.1523/JNEUROSCI.4291-12.2013. J Neurosci. 2013. PMID: 23407949 Free PMC article.
-
Morphology of axonal projections from the high vocal center to vocal motor cortex in songbirds.J Comp Neurol. 2012 Aug 15;520(12):2742-56. doi: 10.1002/cne.23084. J Comp Neurol. 2012. PMID: 22684940 Free PMC article.
-
The gain modulation by N-methyl-D-aspartate in the projection neurons of robust nucleus of the arcopallium in adult zebra finches.Neural Plast. 2012;2012:931780. doi: 10.1155/2012/931780. Epub 2012 May 20. Neural Plast. 2012. PMID: 22685680 Free PMC article.
-
Motor cortex analogue neurons in songbirds utilize Kv3 channels to generate ultranarrow spikes.Elife. 2023 May 9;12:e81992. doi: 10.7554/eLife.81992. Elife. 2023. PMID: 37158590 Free PMC article.
-
Cellular transcriptomics reveals evolutionary identities of songbird vocal circuits.Science. 2021 Feb 12;371(6530):eabd9704. doi: 10.1126/science.abd9704. Science. 2021. PMID: 33574185 Free PMC article.
References
-
- Bottjer SW, Miesner EA, Arnold AP (1984) Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224: 901-903. - PubMed
-
- Brainard MS, Doupe AJ (2000) Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature 404: 762-766. - PubMed
-
- Chi Z, Margoliash D (2001) Temporal precision and temporal drift in brain and behavior of zebra finch song. Neuron 32: 899-910. - PubMed
-
- Fee MS, Leonardo A (2001) Miniature motorized microdrive and commutator system for chronic neural recordings in small animals. J Neurosci Methods 112: 83-94. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
