Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro
- PMID: 15659603
- PMCID: PMC6725326
- DOI: 10.1523/JNEUROSCI.4166-04.2005
Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro
Abstract
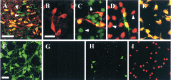
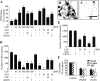
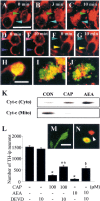
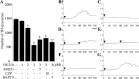
Intranigral injection of the transient receptor potential vanilloid subtype 1 (TRPV1; also known as VR1) agonist capsaicin (CAP) into the rat brain, or treatment of rat mesencephalic cultures with CAP, resulted in cell death of dopaminergic (DA) neurons, as visualized by immunocytochemistry. This in vivo and in vitro effect was ameliorated by the TRPV1 antagonist capsazepine (CZP) or iodo-resiniferatoxin, suggesting the direct involvement of TRPV1 in neurotoxicity. In cultures, both CAP and anandamide (AEA), an endogenous ligand for both TRPV1 and cannabinoid type 1 (CB1) receptors, induced degeneration of DA neurons, increases in intracellular Ca2+ ([Ca2+]i), and mitochondrial damage, which were inhibited by CZP, the CB1 antagonist N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) or the intracellular Ca2+ chelator BAPTA/AM. We also found that CAP or AEA increased mitochondrial cytochrome c release as well as immunoreactivity to cleaved caspase-3 and that the caspase-3 inhibitor z-Asp-Glu-Val-Asp-fmk protected DA neurons from CAP- or AEA-induced neurotoxicity. Additional studies demonstrated that treatment of mesencephalic cultures with CB1 receptor agonist (6aR)-trans 3-(1,1-dimethylheptyl)-6a,7,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d] pyran-9-methanol (HU210) also produced degeneration of DA neurons and increases in [Ca2+]i, which were inhibited by AM251 and BAPTA/AM. The CAP-, AEA-, or HU210-induced increases in [Ca2+]i were dependent on extracellular Ca2+, with significantly different patterns of Ca2+ influx. Surprisingly, CZP and AM251 reversed HU210- or CAP-induced neurotoxicity by inhibiting Ca2+ influx, respectively, suggesting the existence of functional cross talk between TRPV1 and CB1 receptors. To our knowledge, this study is the first to demonstrate that the activation of TRPV1 and/or CB1 receptors mediates cell death of DA neurons. Our findings suggest that these two types of receptors, TRPV1 and CB1, may contribute to neurodegeneration in response to endogenous ligands such as AEA.
Figures

Similar articles
-
Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release.J Immunol. 2006 Oct 1;177(7):4322-9. doi: 10.4049/jimmunol.177.7.4322. J Immunol. 2006. PMID: 16982866
-
Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide.Br J Pharmacol. 2004 Apr;141(7):1118-30. doi: 10.1038/sj.bjp.0705711. Epub 2004 Mar 8. Br J Pharmacol. 2004. PMID: 15006899 Free PMC article.
-
Transient receptor potential vanilloid subtype 1 contributes to mesencephalic dopaminergic neuronal survival by inhibiting microglia-originated oxidative stress.Brain Res Bull. 2012 Nov 1;89(3-4):92-6. doi: 10.1016/j.brainresbull.2012.07.001. Epub 2012 Jul 13. Brain Res Bull. 2012. PMID: 22796104
-
Roles of transient receptor potential vanilloid subtype 1 and cannabinoid type 1 receptors in the brain: neuroprotection versus neurotoxicity.Mol Neurobiol. 2007 Jun;35(3):245-54. doi: 10.1007/s12035-007-0030-1. Mol Neurobiol. 2007. PMID: 17917113 Review.
-
Anandamide-Mediated Modulation of Nociceptive Transmission at the Spinal Cord Level.Physiol Res. 2024 Aug 30;73(S1):S435-S448. doi: 10.33549/physiolres.935371. Epub 2024 Jul 2. Physiol Res. 2024. PMID: 38957948 Free PMC article. Review.
Cited by
-
Nicotine from edible Solanaceae and risk of Parkinson disease.Ann Neurol. 2013 Sep;74(3):472-7. doi: 10.1002/ana.23884. Epub 2013 May 9. Ann Neurol. 2013. PMID: 23661325 Free PMC article.
-
New Frontiers on ER Stress Modulation: Are TRP Channels the Leading Actors?Int J Mol Sci. 2022 Dec 22;24(1):185. doi: 10.3390/ijms24010185. Int J Mol Sci. 2022. PMID: 36613628 Free PMC article. Review.
-
Capsaicin: Physicochemical properties, cutaneous reactions and potential applications in painful and inflammatory conditions.Exp Ther Med. 2019 Aug;18(2):916-925. doi: 10.3892/etm.2019.7513. Epub 2019 Apr 19. Exp Ther Med. 2019. PMID: 31384324 Free PMC article. Review.
-
The Role of TRP Channels and PMCA in Brain Disorders: Intracellular Calcium and pH Homeostasis.Front Cell Dev Biol. 2021 Jan 28;9:584388. doi: 10.3389/fcell.2021.584388. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33585474 Free PMC article. Review.
-
Promising cannabinoid-based therapies for Parkinson's disease: motor symptoms to neuroprotection.Mol Neurodegener. 2015 Apr 8;10:17. doi: 10.1186/s13024-015-0012-0. Mol Neurodegener. 2015. PMID: 25888232 Free PMC article. Review.
References
-
- Campbell VA (2001) Tetrahydrocannabinol-induced apoptosis of cultured cortical neurones is associated with cytochrome c release and caspase-3 activation. Neuropharmacology 40: 702-709. - PubMed
-
- Caterina MJ, Julius D (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487-517. - PubMed
-
- Cernak I, Vink R, Natale J, Stoica B, Lea IVPM, Movsesyan V, Ahmed F, Knoblach SM, Fricke ST, Faden AI (2004) The “dark side” of endocannabinoids: a neurotoxic role for anandamide. J Cereb Blood Flow Metab 24: 564-578. - PubMed
-
- Choi SH, Lee DY, Ryu JK, Kim J, Joe EH, Jin BK (2003a) Thrombin induces nigral dopaminergic neurodegeneration in vivo by altering expression of death-related proteins. Neurobiol Dis 14: 181-193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
