Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42
- PMID: 15659604
- PMCID: PMC6725334
- DOI: 10.1523/JNEUROSCI.4276-04.2005
Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42
Abstract
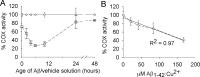
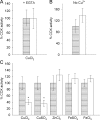
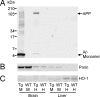
In studies of Alzheimer's disease pathogenesis there is an increasing focus on mechanisms of intracellular amyloid-beta (Abeta) generation and toxicity. Here we investigated the inhibitory potential of the 42 amino acid Abeta peptide (Abeta1-42) on activity of electron transport chain enzyme complexes in human mitochondria. We found that synthetic Abeta1-42 specifically inhibited the terminal complex cytochrome c oxidase (COX) in a dose-dependent manner that was dependent on the presence of Cu2+ and specific "aging" of the Abeta1-42 solution. Maximal COX inhibition occurred when using Abeta1-42 solutions aged for 3-6 h at 30 degrees C. The level of Abeta1-42-mediated COX inhibition increased with aging time up to approximately 6 h and then declined progressively with continued aging to 48 h. Photo-induced cross-linking of unmodified proteins followed by SDS-PAGE analysis revealed dimeric Abeta as the only Abeta species to provide significant temporal correlation with the observed COX inhibition. Analysis of brain and liver from an Alzheimer's model mouse (Tg2576) revealed abundant Abeta immunoreactivity within the brain mitochondria fraction. Our data indicate that endogenous Abeta is associated with brain mitochondria and that Abeta1-42, possibly in its dimeric conformation, is a potent inhibitor of COX, but only when in the presence of Cu2+. We conclude that Cu2+-dependent Abeta-mediated inhibition of COX may be an important contributor to the neurodegeneration process in Alzheimer's disease.
Figures


Similar articles
-
Copper-dependent inhibition of cytochrome c oxidase by Abeta(1-42) requires reduced methionine at residue 35 of the Abeta peptide.J Neurochem. 2006 Oct;99(1):226-36. doi: 10.1111/j.1471-4159.2006.04050.x. J Neurochem. 2006. PMID: 16987248
-
Neurotoxic Abeta peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro.J Neurochem. 2001 Feb;76(4):1050-6. doi: 10.1046/j.1471-4159.2001.00112.x. J Neurochem. 2001. PMID: 11181824
-
Substrate-bound beta-amyloid peptides inhibit cell adhesion and neurite outgrowth in primary neuronal cultures.J Neurochem. 2000 Mar;74(3):1122-30. doi: 10.1046/j.1471-4159.2000.741122.x. J Neurochem. 2000. PMID: 10693944
-
ApoE and Abeta1-42 interactions: effects of isoform and conformation on structure and function.J Mol Neurosci. 2004;23(3):235-46. doi: 10.1385/JMN:23:3:235. J Mol Neurosci. 2004. PMID: 15181252 Review.
-
[Brain Function and Pathophysiology Focused on Zn2+ Dynamics].Yakugaku Zasshi. 2022;142(8):855-866. doi: 10.1248/yakushi.22-00074. Yakugaku Zasshi. 2022. PMID: 35908946 Review. Japanese.
Cited by
-
Anti-amyloid Aggregation Activity of Natural Compounds: Implications for Alzheimer's Drug Discovery.Mol Neurobiol. 2016 Aug;53(6):3565-3575. doi: 10.1007/s12035-015-9301-4. Epub 2015 Jun 23. Mol Neurobiol. 2016. PMID: 26099310 Review.
-
Amyloid-β peptide binds to cytochrome C oxidase subunit 1.PLoS One. 2012;7(8):e42344. doi: 10.1371/journal.pone.0042344. Epub 2012 Aug 21. PLoS One. 2012. PMID: 22927926 Free PMC article.
-
Amyloid beta-induced glycogen synthase kinase 3β phosphorylated VDAC1 in Alzheimer's disease: implications for synaptic dysfunction and neuronal damage.Biochim Biophys Acta. 2013 Dec;1832(12):1913-21. doi: 10.1016/j.bbadis.2013.06.012. Epub 2013 Jun 28. Biochim Biophys Acta. 2013. PMID: 23816568 Free PMC article. Review.
-
Mitochondrial oxidative damage in aging and Alzheimer's disease: implications for mitochondrially targeted antioxidant therapeutics.J Biomed Biotechnol. 2006;2006(3):31372. doi: 10.1155/JBB/2006/31372. J Biomed Biotechnol. 2006. PMID: 17047303 Free PMC article.
-
Mitochondria are related to synaptic pathology in Alzheimer's disease.Int J Alzheimers Dis. 2011;2011:305395. doi: 10.4061/2011/305395. Epub 2011 Sep 12. Int J Alzheimers Dis. 2011. PMID: 21922047 Free PMC article.
References
-
- Anderson MF, Sims NR (2000) Improved recovery of highly enriched mitochondrial fractions from small brain tissue samples. Brain Res Protoc 5: 95-101. - PubMed
-
- Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NME, Romano DM, Hartshorn MA, Tanzi RE, Bush AI (1998) Dramatic aggregation of Alzheimer Aβ by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem 273: 12817-12826. - PubMed
-
- Atwood CS, Perry G, Zeng H, Kato Y, Jones WD, Ling K-Q, Huang X, Moir RD, Wang D, Sayre LM, Smith MA, Chen SG, Bush AI (2004) Copper mediates dityrosine cross-linking of Alzheimer's amyloid-β. Biochemistry 43: 560-568. - PubMed
-
- Bitan G, Lomakin A, Teplow DB (2001) Amyloid β-protein oligomerization: prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. J Biol Chem 276: 35176-35184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
