Expression of stathmin, a developmentally controlled cytoskeleton-regulating molecule, in demyelinating disorders
- PMID: 15659612
- PMCID: PMC6725324
- DOI: 10.1523/JNEUROSCI.4174-04.2005
Expression of stathmin, a developmentally controlled cytoskeleton-regulating molecule, in demyelinating disorders
Abstract
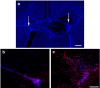
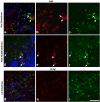
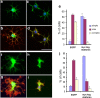
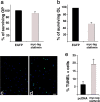
Understanding the biological relevance of reexpression of developmental molecules in pathological conditions is crucial for the development of new therapies. In this study, we report the increased expression of stathmin, a developmentally regulated tubulin-binding protein, in the brains of patients with multiple sclerosis (MS). In physiological conditions, stathmin immunoreactivity was observed in polysialic acid-neural cell adhesion molecule-positive migratory progenitors in the subventricular zone, and its expression progressively decreased as the cells matured into oligodendrocytes (OLs). In MS patients, however, stathmin levels were elevated in 2',3'-cyclic nucleotide 3'-phosphodiesterase-positive OLs, in 10 of 10 bioptic samples analyzed. Increased levels of stathmin were confirmed by Western blot analysis of normal-appearing white matter samples from MS brains. In addition, using mass spectrometry, stathmin was identified as the main component of a specific myelin protein fraction consistently increased in MS preparations compared with controls. To test the biological relevance of increased stathmin levels, primary OL progenitors were transfected using a myc-tagged stathmin cDNA and were allowed to differentiate. Consistent with a distinct role played by this molecule in cells of the OL lineage at different developmental stages, transient transfection in progenitors favored the bipolar migratory phenotype but did not affect survival. However, sustained stathmin levels in differentiating OLs, because of overexpression, resulted in enhanced apoptotic susceptibility. We conclude that stathmin expression in demyelinating disorders could have a dual role. On one hand, by favoring the migratory phenotype of progenitors, it may promote myelin repair. On the other hand, stathmin in mature OLs may indicate cell stress and possibly affect survival.
Figures




Similar articles
-
Oligodendrogliopathy in Multiple Sclerosis: Low Glycolytic Metabolic Rate Promotes Oligodendrocyte Survival.J Neurosci. 2016 Apr 27;36(17):4698-707. doi: 10.1523/JNEUROSCI.4077-15.2016. J Neurosci. 2016. PMID: 27122029 Free PMC article.
-
AATYK is a Novel Regulator of Oligodendrocyte Differentiation and Myelination.Neurosci Bull. 2018 Jun;34(3):527-533. doi: 10.1007/s12264-018-0218-6. Epub 2018 Mar 19. Neurosci Bull. 2018. PMID: 29556912 Free PMC article.
-
Polysialylation at Early Stages of Oligodendrocyte Differentiation Promotes Myelin Repair.J Neurosci. 2017 Aug 23;37(34):8131-8141. doi: 10.1523/JNEUROSCI.1147-17.2017. Epub 2017 Jul 31. J Neurosci. 2017. PMID: 28760868 Free PMC article.
-
Hormonal Regulation of Oligodendrogenesis II: Implications for Myelin Repair.Biomolecules. 2021 Feb 16;11(2):290. doi: 10.3390/biom11020290. Biomolecules. 2021. PMID: 33669242 Free PMC article. Review.
-
The life, death, and replacement of oligodendrocytes in the adult CNS.J Neurochem. 2008 Oct;107(1):1-19. doi: 10.1111/j.1471-4159.2008.05570.x. Epub 2008 Jul 15. J Neurochem. 2008. PMID: 18643793 Review.
Cited by
-
Peptidylarginine deiminase 2 (PAD2) overexpression in transgenic mice leads to myelin loss in the central nervous system.Dis Model Mech. 2008 Nov-Dec;1(4-5):229-40. doi: 10.1242/dmm.000729. Epub 2008 Nov 6. Dis Model Mech. 2008. PMID: 19093029 Free PMC article.
-
Increased stathmin1 expression in the dentate gyrus of mice causes abnormal axonal arborizations.PLoS One. 2010 Jan 6;5(1):e8596. doi: 10.1371/journal.pone.0008596. PLoS One. 2010. PMID: 20062533 Free PMC article.
-
Evaluating epigenetic landmarks in the brain of multiple sclerosis patients: a contribution to the current debate on disease pathogenesis.Prog Neurobiol. 2008 Dec 11;86(4):368-78. doi: 10.1016/j.pneurobio.2008.09.012. Epub 2008 Sep 26. Prog Neurobiol. 2008. PMID: 18930111 Free PMC article. Review.
-
The cancer drug tamoxifen: a potential therapeutic treatment for spinal cord injury.J Neurotrauma. 2014 Feb 1;31(3):268-83. doi: 10.1089/neu.2013.3108. Epub 2013 Dec 11. J Neurotrauma. 2014. PMID: 24004276 Free PMC article.
-
Epigenetic memory loss in aging oligodendrocytes in the corpus callosum.Neurobiol Aging. 2008 Mar;29(3):452-63. doi: 10.1016/j.neurobiolaging.2006.10.026. Epub 2006 Dec 19. Neurobiol Aging. 2008. PMID: 17182153 Free PMC article.
References
-
- Almeida A, Delgado-Esteban M, Bolanos JP, Medina JM (2002) Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primaryculture. J Neurochem 81: 207-217. - PubMed
-
- Amat JA, Fields KL, Schubart UK (1991) Distribution of phosphoprotein p19 in rat brain during ontogeny: stage-specific expression in neurons and glia. Dev Brain Res 60: 205-218. - PubMed
-
- Anderson DJ, Axel R (1985) Molecular probes for the development and plasticity of neural crest derivatives. Cell 42: 649-662. - PubMed
-
- Bakshi R, Miletich RS, Kinkel PR, Emmet ML, Kinkel WR (1998) Highresolution fluorodeoxyglucose positron emission tomography shows both global and regional cerebral hypometabolism in multiple sclerosis. J Neuroimaging 8: 228-234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
