Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons
- PMID: 15659614
- PMCID: PMC6725322
- DOI: 10.1523/JNEUROSCI.3909-04.2005
Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons
Abstract
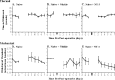
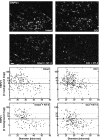
Neurotrophin-3 (NT-3) negatively modulates nerve growth factor (NGF) receptor expression and associated nociceptive phenotype in intact neurons, suggesting a beneficial role in treating aspects of neuropathic pain mediated by NGF. We report that NT-3 is effective at suppressing thermal hyperalgesia associated with chronic constriction injury (CCI); however, NT-3 does not alter the mechanical hypersensitivity that also develops with CCI. Thermal hyperalgesia is critically linked to expression and activation of the capsaicin receptor, transient receptor potential vanilloid receptor-1 (TRPV1). Thus, its modulation by NT-3 after CCI was examined. CCI results in elevated TRPV1 expression at both the mRNA and protein levels in predominantly small-to-medium neurons, with the percentage of neurons expressing TRPV1 remaining unchanged at approximately 56%. Attenuation of thermal hyperalgesia mediated by NT-3 correlates with decreased TRPV1 expression such that only approximately 26% of neurons ipsilateral to CCI expressed detectable TRPV1 mRNA. NT-3 effected a decrease in expression of the activated component of the signaling pathway linked to regulation of TRPV1 expression, phospho-p38 MAPK (Ji et al., 2002), in neurons ipsilateral to CCI. Exogenous NT-3 could both prevent the onset of thermal hyperalgesia and reverse established thermal hyperalgesia and elevated TRPV1 expression 1 week after CCI. Continuous infusion is required for suppression of both thermal hyperalgesia and TRPV1 expression, because removal of NT-3 resulted in a prompt reestablishment of the hyperalgesic state and corresponding CCI-associated TRPV1 phenotype. In conclusion, although NGF drives inflammation-associated thermal hyperalgesia via its regulation of TRPV1 expression, NT-3 is now identified as a potent negative modulator of this state.
Figures





References
-
- Bennett GJ, Xie Y-K (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33: 87-107. - PubMed
-
- Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB (2000) Potent analgesic effects of GDNF in neuropathic pain states. Science 290: 124-127. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816-824. - PubMed
-
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306-313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
