VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract
- PMID: 15659637
- PMCID: PMC548828
- DOI: 10.1105/tpc.104.027631
VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract
Abstract
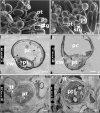
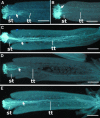
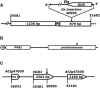
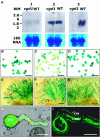
In flowering plants, penetration of the pollen tube through stigma, style, and transmitting tract is essential for delivery of sperm nuclei to the egg cells embedded deeply within female tissues. Despite its importance in plant reproduction, little is known about the underlying molecular mechanisms that regulate the navigation of the pollen tube through the stigma, style, and transmitting tract. Here, we report the identification and characterization of an Arabidopsis thaliana gene, VANGUARD1 (VGD1) that encodes a pectin methylesterase (PME)-homologous protein of 595 amino acids and is required for enhancing the growth of pollen tubes in the style and transmitting tract tissues. VGD1 was expressed specifically in pollen grain and the pollen tube. The VGD1 protein was distributed throughout the pollen grain and pollen tube, including the plasma membrane and cell wall. Functional interruption of VGD1 reduced PME activity in the pollen to 82% of the wild type and greatly retarded the growth of the pollen tube in the style and transmitting tract, resulting in a significant reduction of male fertility. In addition, the vgd1 pollen tubes were unstable and burst more frequently when germinated and grown on in vitro culture medium, compared with wild-type pollen tubes. Our study suggests that the VGD1 product is required for growth of the pollen tube, possibly via modifying the cell wall and enhancing the interaction of the pollen tube with the female style and transmitting tract tissues.
Figures



Similar articles
-
Pollen-specific gene SKU5-SIMILAR 13 enhances growth of pollen tubes in the transmitting tract in Arabidopsis.J Exp Bot. 2022 Jan 27;73(3):696-710. doi: 10.1093/jxb/erab448. J Exp Bot. 2022. PMID: 34626184
-
PECTIN METHYLESTERASE48 is involved in Arabidopsis pollen grain germination.Plant Physiol. 2015 Feb;167(2):367-80. doi: 10.1104/pp.114.250928. Epub 2014 Dec 18. Plant Physiol. 2015. PMID: 25524442 Free PMC article.
-
The cell wall pectic polymer rhamnogalacturonan-II is required for proper pollen tube elongation: implications of a putative sialyltransferase-like protein.Ann Bot. 2014 Oct;114(6):1177-88. doi: 10.1093/aob/mcu093. Epub 2014 May 13. Ann Bot. 2014. PMID: 24825296 Free PMC article.
-
Evolutionary origins of pectin methylesterase genes associated with novel aspects of angiosperm pollen tube walls.Biochem Biophys Res Commun. 2017 Jun 3;487(3):509-516. doi: 10.1016/j.bbrc.2017.04.027. Epub 2017 Apr 7. Biochem Biophys Res Commun. 2017. PMID: 28396152
-
How many receptor-like kinases are required to operate a pollen tube.Curr Opin Plant Biol. 2018 Feb;41:73-82. doi: 10.1016/j.pbi.2017.09.008. Epub 2017 Oct 6. Curr Opin Plant Biol. 2018. PMID: 28992536 Review.
Cited by
-
Transcript profiles of maize embryo sacs and preliminary identification of genes involved in the embryo sac-pollen tube interaction.Front Plant Sci. 2014 Dec 17;5:702. doi: 10.3389/fpls.2014.00702. eCollection 2014. Front Plant Sci. 2014. PMID: 25566277 Free PMC article.
-
Organ evolution in angiosperms driven by correlated divergences of gene sequences and expression patterns.Plant Cell. 2013 Jan;25(1):71-82. doi: 10.1105/tpc.112.106716. Epub 2013 Jan 22. Plant Cell. 2013. PMID: 23341336 Free PMC article.
-
RNAi mediated silencing of a wall associated kinase, OsiWAK1 in Oryza sativa results in impaired root development and sterility due to anther indehiscence: Wall Associated Kinases from Oryza sativa.Physiol Mol Biol Plants. 2011 Mar;17(1):65-77. doi: 10.1007/s12298-011-0050-1. Epub 2011 Jan 21. Physiol Mol Biol Plants. 2011. PMID: 23572996 Free PMC article.
-
Pectin Methylesterases: Cell Wall Remodeling Proteins Are Required for Plant Response to Heat Stress.Front Plant Sci. 2018 Nov 6;9:1612. doi: 10.3389/fpls.2018.01612. eCollection 2018. Front Plant Sci. 2018. PMID: 30459794 Free PMC article. Review.
-
The evolving views of the simplest pectic polysaccharides: homogalacturonan.Plant Cell Rep. 2022 Nov;41(11):2111-2123. doi: 10.1007/s00299-022-02909-3. Epub 2022 Aug 20. Plant Cell Rep. 2022. PMID: 35986766 Review.
References
-
- Bordenave, M., and Goldberg, R. (1993). Purification and characterization of pectin methylesterases from mung bean hypocotyl cell walls. Phytochemistry 33, 999–1003.
-
- Catoire, L., Pierron, M., Morvan, C., du Penhoat, C.H., and Goldberg, R. (1998). Investigation of the action patterns of pectin methylesterase isoforms through kinetic analyses and NMR spectroscopy. J. Biol. Chem. 50, 33150–33156. - PubMed
-
- Denés, J.M., Naron, A., Renard, C.M., Pean, C., and Drilleau, J.F. (2000). Different action patterns for apple pectin methylesterase at pH 7.0 and 4.5. Carbohydr. Res. 327, 385–393. - PubMed
-
- Elleman, C.J., Franklin Tong, V., and Dickinson, H.G. (1992). Pollination in species with dry stigmas: The nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytol. 121, 413–424. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

