Postprenylation CAAX processing is required for proper localization of Ras but not Rho GTPases
- PMID: 15659645
- PMCID: PMC1073645
- DOI: 10.1091/mbc.e04-11-0960
Postprenylation CAAX processing is required for proper localization of Ras but not Rho GTPases
Abstract
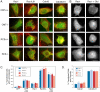
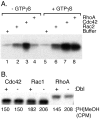
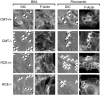
The CAAX motif at the C terminus of most monomeric GTPases is required for membrane targeting because it signals for a series of three posttranslational modifications that include isoprenylation, endoproteolytic release of the C-terminal- AAX amino acids, and carboxyl methylation of the newly exposed isoprenylcysteine. The individual contributions of these modifications to protein trafficking and function are unknown. To address this issue, we performed a series of experiments with mouse embryonic fibroblasts (MEFs) lacking Rce1 (responsible for removal of the -AAX sequence) or Icmt (responsible for carboxyl methylation of the isoprenylcysteine). In MEFs lacking Rce1 or Icmt, farnesylated Ras proteins were mislocalized. In contrast, the intracellular localizations of geranylgeranylated Rho GTPases were not perturbed. Consistent with the latter finding, RhoGDI binding and actin remodeling were normal in Rce1- and Icmt-deficient cells. Swapping geranylgeranylation for farnesylation on Ras proteins or vice versa on Rho proteins reversed the differential sensitivities to Rce1 and Icmt deficiency. These results suggest that postprenylation CAAX processing is required for proper localization of farnesylated Ras but not geranygeranylated Rho proteins.
Figures






References
-
- Andres, D. A., Seabra, M. C., Brown, M. S., Armstrong, S. A., Smeland, T. E., Cremers, F. P., and Goldstein, J. L. (1993). cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell 73, 1091–1099. - PubMed
-
- Bergo, M. O., Leung, G. K., Ambroziak, P., Otto, J. C., Casey, P. J., Gomes, A. Q., Seabra, M. C., and Young, S. G. (2001). Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J. Biol. Chem. 276, 5841–5845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

