Yos1p is a novel subunit of the Yip1p-Yif1p complex and is required for transport between the endoplasmic reticulum and the Golgi complex
- PMID: 15659647
- PMCID: PMC1073651
- DOI: 10.1091/mbc.e04-10-0873
Yos1p is a novel subunit of the Yip1p-Yif1p complex and is required for transport between the endoplasmic reticulum and the Golgi complex
Abstract
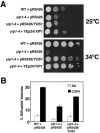
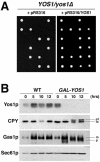
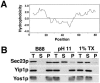
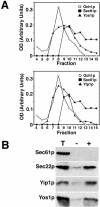
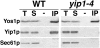
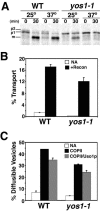
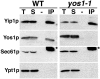
Yeast Yip1p is a member of a conserved family of transmembrane proteins that interact with Rab GTPases. Previous studies also have indicated a role for Yip1p in the biogenesis of endoplasmic reticulum (ER)-derived COPII transport vesicles. In this report, we describe the identification and characterization of the uncharacterized open reading frame YER074W-A as a novel multicopy suppressor of the thermosensitive yip1-4 strain. We have termed this gene Yip One Suppressor 1 (YOS1). Yos1p is essential for growth and for function of the secretory pathway; depletion or inactivation of Yos1p blocks transport between the ER and the Golgi complex. YOS1 encodes an integral membrane protein of 87 amino acids that is conserved in eukaryotes. Yos1p localizes to ER and Golgi membranes and is efficiently packaged into ER-derived COPII transport vesicles. Yos1p associates with Yip1p and Yif1p, indicating Yos1p is a novel subunit of the Yip1p-Yif1p complex.
Figures



Similar articles
-
The Yip1p.Yif1p complex is required for the fusion competence of endoplasmic reticulum-derived vesicles.J Biol Chem. 2003 May 30;278(22):19878-84. doi: 10.1074/jbc.M302406200. Epub 2003 Mar 25. J Biol Chem. 2003. PMID: 12657649
-
Multicopy suppressor analysis of thermosensitive YIP1 alleles implicates GOT1 in transport from the ER.J Cell Sci. 2009 May 15;122(Pt 10):1540-50. doi: 10.1242/jcs.042457. Epub 2009 Apr 21. J Cell Sci. 2009. PMID: 19383723 Free PMC article.
-
Genetic analysis of yeast Yip1p function reveals a requirement for Golgi-localized rab proteins and rab-Guanine nucleotide dissociation inhibitor.Genetics. 2004 Dec;168(4):1827-41. doi: 10.1534/genetics.104.032888. Genetics. 2004. PMID: 15611160 Free PMC article.
-
Making COPII coats.Cell. 2007 Jun 29;129(7):1251-2. doi: 10.1016/j.cell.2007.06.015. Cell. 2007. PMID: 17604713 Review.
-
An Overview of Protein Secretion in Yeast and Animal Cells.Methods Mol Biol. 2017;1662:1-17. doi: 10.1007/978-1-4939-7262-3_1. Methods Mol Biol. 2017. PMID: 28861813 Review.
Cited by
-
Expression characterization and transcription regulation analysis of porcine Yip1 domain family member 3 gene.Asian-Australas J Anim Sci. 2020 Mar;33(3):398-407. doi: 10.5713/ajas.19.0076. Epub 2019 Jul 2. Asian-Australas J Anim Sci. 2020. PMID: 31480180 Free PMC article.
-
An abscisic acid-induced protein, HVA22, inhibits gibberellin-mediated programmed cell death in cereal aleurone cells.Plant Physiol. 2008 Aug;147(4):1710-22. doi: 10.1104/pp.108.120238. Epub 2008 Jun 26. Plant Physiol. 2008. PMID: 18583533 Free PMC article.
-
GOLGI TRANSPORT 1B Regulates Protein Export from the Endoplasmic Reticulum in Rice Endosperm Cells.Plant Cell. 2016 Nov;28(11):2850-2865. doi: 10.1105/tpc.16.00717. Epub 2016 Nov 1. Plant Cell. 2016. PMID: 27803308 Free PMC article.
-
Genome-wide association identifies multiple genomic regions associated with susceptibility to and control of ovine lentivirus.PLoS One. 2012;7(10):e47829. doi: 10.1371/journal.pone.0047829. Epub 2012 Oct 17. PLoS One. 2012. PMID: 23082221 Free PMC article.
-
Post-translational regulation of sucrose transporters by direct protein-protein interactions.Front Plant Sci. 2013 Jul 2;4:237. doi: 10.3389/fpls.2013.00237. eCollection 2013. Front Plant Sci. 2013. PMID: 23847641 Free PMC article.
References
-
- Ausubel, R. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A., and Struhl, K. (1987). Current Protocols in Molecular Biology, New York, Greene Publishing Associates and Wiley-Interscience.
-
- Baker, D., Hicke, L., Rexach, M., Schleyer, M., and Schekman, R. (1988). Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell 54, 335–344. - PubMed
-
- Barrowman, J., Wang, W., Zhang, Y., and Ferro-Novick, S. (2003). The Yip1p·Yif1p complex is required for the fusion competence of endoplasmic reticulum-derived vesicles. J. Biol. Chem. 278, 19878–19884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

