Genome-wide expression analysis indicates that FNR of Escherichia coli K-12 regulates a large number of genes of unknown function
- PMID: 15659690
- PMCID: PMC545700
- DOI: 10.1128/JB.187.3.1135-1160.2005
Genome-wide expression analysis indicates that FNR of Escherichia coli K-12 regulates a large number of genes of unknown function
Abstract
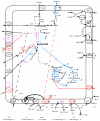
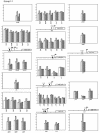
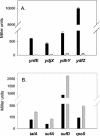
The major regulator controlling the physiological switch between aerobic and anaerobic growth conditions in Escherichia coli is the DNA binding protein FNR. To identify genes controlled by FNR, we used Affymetrix Antisense GeneChips to compare global gene expression profiles from isogenic MG1655 wild-type and Deltafnr strains grown in glucose minimal media under aerobic or anaerobic conditions. We found that 297 genes contained within 184 operons were regulated by FNR and/or by O2 levels. The expression of many genes known to be involved in anaerobic respiration and fermentation was increased under anaerobic growth conditions, while that of genes involved in aerobic respiration and the tricarboxylic acid cycle were repressed as expected. The expression of nine operons associated with acid resistance was also increased under anaerobic growth conditions, which may reflect the production of acidic fermentation products. Ninety-one genes with no presently defined function were also altered in expression, including seven of the most highly anaerobically induced genes, six of which we found to be directly regulated by FNR. Classification of the 297 genes into eight groups by k-means clustering analysis indicated that genes with common gene expression patterns also had a strong functional relationship, providing clues for studying the function of unknown genes in each group. Six of the eight groups showed regulation by FNR; while some expression groups represent genes that are simply activated or repressed by FNR, others, such as those encoding functions for chemotaxis and motility, showed a more complex pattern of regulation. A computer search for FNR DNA binding sites within predicted promoter regions identified 63 new sites for 54 genes. We suggest that E. coli MG1655 has a larger metabolic potential under anaerobic conditions than has been previously recognized.
Figures
















Similar articles
-
Novel FNR-Dependent Oxygen-Responsive Promoters in Escherichia coli: Design, Characterization, and Metabolic Engineering Applications.ACS Synth Biol. 2025 Jul 18;14(7):2832-2844. doi: 10.1021/acssynbio.5c00215. Epub 2025 Jun 21. ACS Synth Biol. 2025. PMID: 40542734
-
Fumarate and nitrate reduction (FNR) dependent activation of the Escherichia coli anaerobic ribonucleotide reductase nrdDG promoter.Int Microbiol. 2008 Mar;11(1):49-56. Int Microbiol. 2008. PMID: 18683632
-
Regulation of the Escherichia coli ydhY-T operon in the presence of alternative electron acceptors.Microbiology (Reading). 2017 Apr;163(4):584-594. doi: 10.1099/mic.0.000445. Epub 2017 Apr 18. Microbiology (Reading). 2017. PMID: 28218056
-
FNR and its role in oxygen-regulated gene expression in Escherichia coli.FEMS Microbiol Rev. 1990 Aug;6(4):399-428. doi: 10.1111/j.1574-6968.1990.tb04109.x. FEMS Microbiol Rev. 1990. PMID: 2248796 Review.
-
Cellular and molecular physiology of Escherichia coli in the adaptation to aerobic environments.J Biochem. 1996 Dec;120(6):1055-63. doi: 10.1093/oxfordjournals.jbchem.a021519. J Biochem. 1996. PMID: 9010748 Review.
Cited by
-
Iron limitation indirectly reduces the Escherichia coli torCAD operon expression by a reduction of molybdenum cofactor availability.Microbiol Spectr. 2024 Feb 6;12(2):e0348023. doi: 10.1128/spectrum.03480-23. Epub 2024 Jan 9. Microbiol Spectr. 2024. PMID: 38193660 Free PMC article.
-
Anaerobic gene expression in Staphylococcus aureus.J Bacteriol. 2007 Jun;189(11):4275-89. doi: 10.1128/JB.00081-07. Epub 2007 Mar 23. J Bacteriol. 2007. PMID: 17384184 Free PMC article.
-
The HU regulon is composed of genes responding to anaerobiosis, acid stress, high osmolarity and SOS induction.PLoS One. 2009;4(2):e4367. doi: 10.1371/journal.pone.0004367. Epub 2009 Feb 4. PLoS One. 2009. PMID: 19194530 Free PMC article.
-
The response of Sphingopyxis granuli strain TFA to the hostile anoxic condition.Sci Rep. 2019 Apr 18;9(1):6297. doi: 10.1038/s41598-019-42768-9. Sci Rep. 2019. PMID: 31000749 Free PMC article.
-
The influence of repressor DNA binding site architecture on transcriptional control.mBio. 2014 Aug 26;5(5):e01684-14. doi: 10.1128/mBio.01684-14. mBio. 2014. PMID: 25161193 Free PMC article.
References
-
- Bhriain, N. N., C. J. Dorman, and C. F. Higgins. 1989. An overlap between osmotic and anaerobic stress responses: a potential role for DNA supercoiling in the coordinate regulation of gene expression. Mol. Microbiol. 3:933-942. - PubMed
-
- Bilous, P. T., S. T. Cole, W. F. Anderson, and J. H. Weiner. 1988. Nucleotide sequence of the dmsABC operon encoding the anaerobic dimethylsulphoxide reductase of Escherichia coli. Mol. Microbiol. 2:785-795. - PubMed
-
- Birkmann, A., F. Zinoni, G. Sawers, and A. Bock. 1987. Factors affecting transcriptional regulation of the formate-hydrogen-lyase pathway of Escherichia coli. Arch. Microbiol. 148:44-51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

