Characterization of phospholipase activity of the Pseudomonas aeruginosa type III cytotoxin, ExoU
- PMID: 15659695
- PMCID: PMC545705
- DOI: 10.1128/JB.187.3.1192-1195.2005
Characterization of phospholipase activity of the Pseudomonas aeruginosa type III cytotoxin, ExoU
Abstract
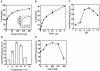
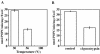
Recombinant ExoU (rExoU) and yeast extract were used to optimize an in vitro phospholipase assay as a basis for identifying the mechanism for enzyme activation and substrate specificity. Our results support a model in which a eukaryotic protein cofactor or complex facilitates the interaction of rExoU with phospholipid substrates.
Figures
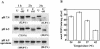
Similar articles
-
Identification of superoxide dismutase as a cofactor for the pseudomonas type III toxin, ExoU.Biochemistry. 2006 Aug 29;45(34):10368-75. doi: 10.1021/bi060788j. Biochemistry. 2006. PMID: 16922513
-
ExoU is a potent intracellular phospholipase.Mol Microbiol. 2004 Sep;53(5):1279-90. doi: 10.1111/j.1365-2958.2004.04194.x. Mol Microbiol. 2004. PMID: 15387809 Review.
-
Mutation of the phospholipase catalytic domain of the Pseudomonas aeruginosa cytotoxin ExoU abolishes colonization promoting activity and reduces corneal disease severity.Exp Eye Res. 2007 Dec;85(6):799-805. doi: 10.1016/j.exer.2007.08.015. Epub 2007 Aug 29. Exp Eye Res. 2007. PMID: 17905228 Free PMC article.
-
The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU.EMBO J. 2003 Jun 16;22(12):2959-69. doi: 10.1093/emboj/cdg290. EMBO J. 2003. PMID: 12805211 Free PMC article.
-
Pseudomonas aeruginosa Type III Secretory Toxin ExoU and Its Predicted Homologs.Toxins (Basel). 2016 Oct 26;8(11):307. doi: 10.3390/toxins8110307. Toxins (Basel). 2016. PMID: 27792159 Free PMC article. Review.
Cited by
-
A novel phosphatidylinositol 4,5-bisphosphate binding domain mediates plasma membrane localization of ExoU and other patatin-like phospholipases.J Biol Chem. 2015 Jan 30;290(5):2919-37. doi: 10.1074/jbc.M114.611251. Epub 2014 Dec 10. J Biol Chem. 2015. PMID: 25505182 Free PMC article.
-
Purification, characterization, molecular cloning and extracellular production of a phospholipase A1 from Streptomyces albidoflavus NA297.FEBS Open Bio. 2012 Sep 28;2:318-27. doi: 10.1016/j.fob.2012.09.006. Print 2012. FEBS Open Bio. 2012. PMID: 23772365 Free PMC article.
-
Omics approaches in cystic fibrosis research: a focus on oxylipin profiling in airway secretions.Ann N Y Acad Sci. 2012 Jul;1259(1):1-9. doi: 10.1111/j.1749-6632.2012.06580.x. Ann N Y Acad Sci. 2012. PMID: 22758630 Free PMC article. Review.
-
Phosphatidylinositol 4,5-bisphosphate is a novel coactivator of the Pseudomonas aeruginosa cytotoxin ExoU.Infect Immun. 2013 Aug;81(8):2873-81. doi: 10.1128/IAI.00414-13. Epub 2013 May 28. Infect Immun. 2013. PMID: 23716613 Free PMC article.
-
Ubiquitin and ubiquitin-modified proteins activate the Pseudomonas aeruginosa T3SS cytotoxin, ExoU.Mol Microbiol. 2011 Dec;82(6):1454-67. doi: 10.1111/j.1365-2958.2011.07904.x. Epub 2011 Nov 21. Mol Microbiol. 2011. PMID: 22040088 Free PMC article.
References
-
- Clark, J. D., L. L. Lin, R. W. Kriz, C. S. Ramesha, L. A. Sultzman, A. Y. Lin, N. Milona, and J. L. Knopf. 1991. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 65:1043-1051. - PubMed
-
- Coburn, J., A. V. Kane, L. Feig, and D. M. Gill. 1991. Pseudomonas aeruginosa exoenzyme S requires a eukaryotic protein for ADP-ribosyltransferase activity. J. Biol. Chem. 266:6438-6446. - PubMed
-
- Dessen, A., J. Tang, H. Schmidt, M. Stahl, J. D. Clark, J. Seehra, and W. S. Somers. 1999. Crystal structure of human cytosolic phospholipase A(2) reveals a novel topology and catalytic mechanism. Cell 97:349-360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

