Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR
- PMID: 15660124
- PMCID: PMC548662
- DOI: 10.1038/sj.emboj.7600557
Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR
Abstract
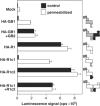
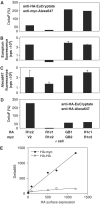
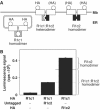
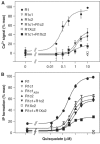
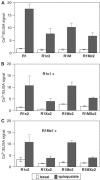
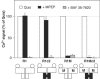
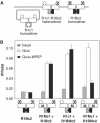
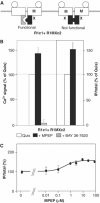
G-protein-coupled receptors (GPCRs) have been shown to form dimers, but the relevance of this phenomenon in G-protein activation is not known. Among the large GPCR family, metabotropic glutamate (mGlu) receptors are constitutive dimers. Here we examined whether both heptahelical domains (HDs) are turned on upon full receptor activation. To that aim, we measured G-protein coupling efficacy of dimeric mGlu receptors in which one subunit bears specific mutations. We show that a mutation in the third intracellular loop (i3 loop) known to prevent G-protein activation in a single subunit decreases coupling efficacy. However, when a single HD is blocked in its inactive state using an inverse agonist, 2-methyl-6-(phenylethynyl)pyridine (MPEP), no decrease in receptor activity is observed. Interestingly, in a receptor dimer in which the subunit that binds MPEP is mutated in its i3 loop, MPEP enhances agonist-induced activity, reflecting a 'better' activation of the adjacent HD. These data are consistent with a model in which a single HD is turned on upon activation of such homodimeric receptors and raise important issues in deciphering the functional role of GPCR dimer formation for G-protein activation.
Figures


References
-
- Angers S, Salahpour A, Bouvier M (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435 - PubMed
-
- Balasubramanian S, Teissere JA, Raju DV, Hall RA (2004) Hetero-oligomerization between GABAA and GABAB receptors regulates GABAB receptor trafficking. J Biol Chem 279: 18840–18850 - PubMed
-
- Baneres J-L, Parello J (2003) Structure-based analysis of GPCR function. Evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. J Mol Biol 329: 815–829 - PubMed

