Inducible dissociation of SCF(Met30) ubiquitin ligase mediates a rapid transcriptional response to cadmium
- PMID: 15660125
- PMCID: PMC548661
- DOI: 10.1038/sj.emboj.7600556
Inducible dissociation of SCF(Met30) ubiquitin ligase mediates a rapid transcriptional response to cadmium
Abstract
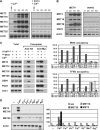
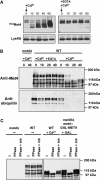
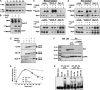
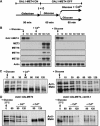
Activity of the Met4 transcription factor is antagonized by the SCF(Met30) ubiquitin ligase by degradation-dependent and degradation-independent mechanisms, in minimal and rich nutrient conditions, respectively. In this study, we show that the heavy metal Cd2+ over-rides both mechanisms to enable rapid Met4-dependent induction of metabolic networks needed for production of the antioxidant and Cd2+-chelating agent glutathione. Cd2+ inhibits SCF(Met30) activity through rapid dissociation of the F-box protein Met30 from the holocomplex. In minimal medium, dissociation of SCF(Met30) complex is sufficient to impair the methionine-induced degradation of Met4. In rich medium, dissociation of the SCF(Met30) complex is accompanied by a deubiquitylation mechanism that rapidly removes inhibitory ubiquitin moieties from Met4. Post-translational control of SCF(Met30) assembly by a physiological stress to allow rapid induction of a protective gene expression program represents a novel mode of regulation in the ubiquitin system.
Figures


References
-
- Blackwell KJ, Tobin JM, Avery SV (1998) Manganese toxicity towards Saccharomyces cerevisiae: dependence on intracellular and extracellular magnesium concentrations. Appl Microbiol Biotechnol 49: 751–757 - PubMed
-
- Cantoni GL (1977) S-adenosylmethionine: present status and future perspectives. In The Biochemistry of S-adenosylmethionine, Salvatore F, Borek E, Zappia V, Williams-Ashman HG, Schlenk F (eds) pp 557–577. New York: Columbia University Press
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

