Examination of Cholesterol oxidase attachment to magnetic nanoparticles
- PMID: 15661076
- PMCID: PMC548673
- DOI: 10.1186/1477-3155-3-1
Examination of Cholesterol oxidase attachment to magnetic nanoparticles
Abstract
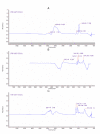
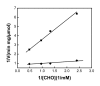
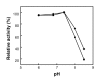
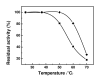
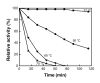
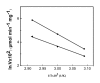
Magnetic nanoparticles (Fe3O4) were synthesized by thermal co-precipitation of ferric and ferrous chlorides. The sizes and structure of the particles were characterized using transmission electron microscopy (TEM). The size of the particles was in the range between 9.7 and 56.4 nm. Cholesterol oxidase (CHO) was successfully bound to the particles via carbodiimide activation. FTIR spectroscopy was used to confirm the binding of CHO to the particles. The binding efficiency was between 98 and 100% irrespective of the amount of particles used. Kinetic studies of the free and bound CHO revealed that the stability and activity of the enzyme were significantly improved upon binding to the nanoparticles. Furthermore, the bound enzyme exhibited a better tolerance to pH, temperature and substrate concentration. The activation energy for free and bound CHO was 13.6 and 9.3 kJ/mol, respectively. This indicated that the energy barrier of CHO activity was reduced upon binding onto Fe3O4 nanoparticles. The improvements observed in activity, stability, and functionality of CHO resulted from structural and conformational changes of the bound enzyme. The study indicates that the stability and activity of CHO could be enhanced via attachment to magnetic nanoparticles and subsequently will contribute to better uses of this enzyme in various biological and clinical applications.
Figures



References
-
- O'Grady K. Biomedical applications of magnetic nanoparticles. J Phys D Appl Phys. 2003;36
-
- Berry C, Curtis ASG. Functionalization of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2003;36:R198–R206. doi: 10.1088/0022-3727/36/13/203. - DOI
-
- Liao MH, Chen DH. Immobilization of yeast alcohol deshydrogenase on magnetic nanoparticles. Biotechnol Lett. 2001;23:1723–1727. doi: 10.1023/A:1012485221802. - DOI
-
- Koneracka' M, Kopcansky' P, Antalik M, Timko M, Ramchand CN, Lobo D, Mehta R, Upadhyay RV. Immobilization of proteins and enzymes to fine magnetic particles. J Magn Magn Mater. 1999;201:427–430. doi: 10.1016/S0304-8853(99)00005-0. - DOI
LinkOut - more resources
Full Text Sources

