Molecular mechanisms of severe acute respiratory syndrome (SARS)
- PMID: 15661082
- PMCID: PMC548145
- DOI: 10.1186/1465-9921-6-8
Molecular mechanisms of severe acute respiratory syndrome (SARS)
Abstract
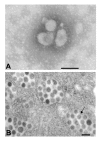
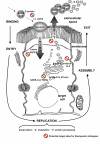
Severe acute respiratory syndrome (SARS) is a new infectious disease caused by a novel coronavirus that leads to deleterious pulmonary pathological features. Due to its high morbidity and mortality and widespread occurrence, SARS has evolved as an important respiratory disease which may be encountered everywhere in the world. The virus was identified as the causative agent of SARS due to the efforts of a WHO-led laboratory network. The potential mutability of the SARS-CoV genome may lead to new SARS outbreaks and several regions of the viral genomes open reading frames have been identified which may contribute to the severe virulence of the virus. With regard to the pathogenesis of SARS, several mechanisms involving both direct effects on target cells and indirect effects via the immune system may exist. Vaccination would offer the most attractive approach to prevent new epidemics of SARS, but the development of vaccines is difficult due to missing data on the role of immune system-virus interactions and the potential mutability of the virus. Even in a situation of no new infections, SARS remains a major health hazard, as new epidemics may arise. Therefore, further experimental and clinical research is required to control the disease.
Figures
References
-
- Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

