Kinetochore-spindle microtubule interactions during mitosis
- PMID: 15661517
- PMCID: PMC8184134
- DOI: 10.1016/j.ceb.2004.12.009
Kinetochore-spindle microtubule interactions during mitosis
Abstract
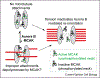
The kinetochore is a proteinaceous structure that assembles onto centromeric DNA and mediates chromosome attachment to microtubules during mitosis. This description is deceivingly simple: recent proteomic studies suggest that the diminutive kinetochores of Saccharomyces cerevisiae are comprised of at least 60 proteins organized into as many as 14 different subcomplexes. Many of these proteins, such as the centromeric histone variant CENP-A, and entire subcomplexes, such as the Ndc80(Hec1) complex, are conserved from yeast to humans despite the diverse nature of the DNA sequences on which they assemble. There have recently been advances in our understanding of the molecular basis of how kinetochores establish dynamic attachments to spindle microtubules, and how these attachments are correctly oriented to ensure segregation of sister chromatids to daughter cells.
Figures
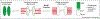

References
-
- McIntosh JR, Grishchuk EL, West RR: Chromosome-microtubule interactions during mitosis. Annu Rev Cell Dev Biol 2002, 18:193–219. - PubMed
-
- Cleveland DW, Mao Y, Sullivan KF: Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 2003, 112:407–421. - PubMed
-
- Lew DJ, Burke DJ: The spindle assembly and spindle position checkpoints. Annu Rev Genet 2003, 37:251–282. - PubMed
-
- Amor DJ, Kalitsis P, Sumer H, ChooKH: Building the centromere: from foundation proteins to 3D organization. Trends Cell Biol 2004, 14:359–368. - PubMed
-
- McAinsh AD, Tytell JD, Sorger PK: Structure, function, and regulation of budding yeast kinetochores. Annu Rev Cell Dev Biol 2003, 19:519–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

