Reduced glycine transporter type 1 expression leads to major changes in glutamatergic neurotransmission of CA1 hippocampal neurones in mice
- PMID: 15661817
- PMCID: PMC1665613
- DOI: 10.1113/jphysiol.2004.080655
Reduced glycine transporter type 1 expression leads to major changes in glutamatergic neurotransmission of CA1 hippocampal neurones in mice
Abstract
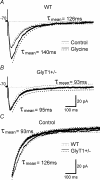
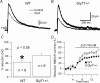
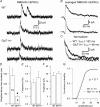
To investigate the effects of persistent elevation of synaptic glycine at Schaffer collateral-CA1 synapses of the hippocampus, we studied the glutamatergic synaptic transmission in acute brain slices from mice with reduced expression of glycine transporter type 1 (GlyT1+/-) as compared to wild type (WT) littermates using whole-cell patch-clamp recordings of CA1 pyramidal cells. We observed faster decay kinetics, reduced ifenprodil sensitivity and increased zinc-induced antagonism in N-methyl-d-aspartate receptor (NMDAR) currents of GlyT1+/- mice. Moreover, the ratio alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR)/NMDAR was decreased in mutants compared to WT. Surprisingly, this change was associated with a reduction in the number of AMPARs expressed at the CA1 synapses in the mutants compared to WT. Overall, these findings highlight the importance of GlyT1 in regulating glutamatergic neurotransmission.
Figures







Similar articles
-
Chronically saturating levels of endogenous glycine disrupt glutamatergic neurotransmission and enhance synaptogenesis in the CA1 region of mouse hippocampus.Synapse. 2011 Nov;65(11):1181-95. doi: 10.1002/syn.20956. Epub 2011 Jun 10. Synapse. 2011. PMID: 21633974
-
Gene knockout of glycine transporter 1: characterization of the behavioral phenotype.Proc Natl Acad Sci U S A. 2004 Jun 1;101(22):8485-90. doi: 10.1073/pnas.0402662101. Epub 2004 May 24. Proc Natl Acad Sci U S A. 2004. PMID: 15159536 Free PMC article.
-
Sustained saturating level of glycine induces changes in NR2B-containing-NMDA receptor localization in the CA1 region of the hippocampus.J Neurochem. 2008 Jun 1;105(6):2454-65. doi: 10.1111/j.1471-4159.2008.05324.x. J Neurochem. 2008. PMID: 18331477
-
Mechanisms of glycine release, which build up synaptic and extrasynaptic glycine levels: the role of synaptic and non-synaptic glycine transporters.Brain Res Bull. 2013 Apr;93:110-9. doi: 10.1016/j.brainresbull.2012.12.002. Epub 2012 Dec 22. Brain Res Bull. 2013. PMID: 23266673 Review.
-
Glycine neurotransmitter transporters: an update.Mol Membr Biol. 2001 Jan-Mar;18(1):13-20. Mol Membr Biol. 2001. PMID: 11396606 Review.
Cited by
-
Altered mnemonic functions and resistance to N-METHYL-d-Aspartate receptor antagonism by forebrain conditional knockout of glycine transporter 1.Neuroscience. 2009 Jun 30;161(2):635-54. doi: 10.1016/j.neuroscience.2009.03.056. Epub 2009 Mar 28. Neuroscience. 2009. PMID: 19332109 Free PMC article.
-
Glycinergic tonic inhibition of hippocampal neurons with depolarizing GABAergic transmission elicits histopathological signs of temporal lobe epilepsy.J Cell Mol Med. 2008 Dec;12(6B):2848-66. doi: 10.1111/j.1582-4934.2008.00357.x. J Cell Mol Med. 2008. PMID: 19210758 Free PMC article.
-
Deletion of forebrain glycine transporter 1 enhances conditioned freezing to a reliable, but not an ambiguous, cue for threat in a conditioned freezing paradigm.Behav Brain Res. 2014 Oct 15;273:1-7. doi: 10.1016/j.bbr.2014.07.018. Epub 2014 Jul 18. Behav Brain Res. 2014. PMID: 25043729 Free PMC article.
-
Synaptic homeostasis in a zebrafish glial glycine transporter mutant.J Neurophysiol. 2008 Oct;100(4):1716-23. doi: 10.1152/jn.90596.2008. Epub 2008 Aug 20. J Neurophysiol. 2008. PMID: 18715895 Free PMC article.
-
D-serine signalling as a prominent determinant of neuronal-glial dialogue in the healthy and diseased brain.J Cell Mol Med. 2008 Oct;12(5B):1872-84. doi: 10.1111/j.1582-4934.2008.00315.x. Epub 2008 Mar 20. J Cell Mol Med. 2008. PMID: 18363840 Free PMC article. Review.
References
-
- Amaral DG, Witter MP. The three dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. 10.1016/0306-4522(89)90424-7. - DOI - PubMed
-
- Berger AJ, Dieudonne S, Ascher P. Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J Neurophysiol. 1998;80:3336–3340. - PubMed
-
- Bogden JD, Troiano RA, Joselow MM. Copper, zinc, magnesium, and calcium in plasma and cerebrospinal fluid of patients with neurological diseases. Clin Chem. 1977;23:485–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

