Identification and characterization of critical cis-acting sequences within the yeast Ty1 retrotransposon
- PMID: 15661848
- PMCID: PMC1370720
- DOI: 10.1261/rna.7860605
Identification and characterization of critical cis-acting sequences within the yeast Ty1 retrotransposon
Abstract
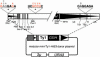
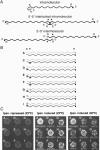
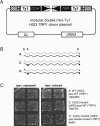
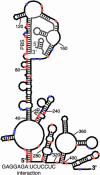
The yeast long terminal repeat (LTR) retrotransposon Ty1, like retroviruses, encodes a terminally redundant RNA, which is packaged into virus-like particles (VLPs) and is converted to a DNA copy by the process of reverse transcription. Mutations predicted to interfere with the priming events during reverse transcription and hence inhibit replication are known to dramatically decrease transposition of Ty1. However, additional cis-acting sequences responsible for Ty1 replication and RNA dimerization and packaging have remained elusive. Here we describe a modular mini-Ty1 element encoding the minimal sequence that can be retrotransposed by the Ty1 proteins, supplied in trans by a helper construct. Using a mutagenic screening strategy, we recovered transposition-deficient modular mini-Ty1-HIS3 elements with mutations in sequences required in cis for Ty1 replication and integration. Two distinct clusters of mutations mapped near the 5'-end of the Ty1 RNA. The clusters define a GAGGAGA sequence at the extreme 5'-end of the Ty1 transcript and a complementary downstream UCUCCUC sequence, 264 nt into the RNA. Disruption of the reverse complementarity of these two sequences decreased transposition and restoration of complementarity rescued transposition to wild-type levels. Ty1 cDNA was reduced in cells expressing RNAs with mutations in either of these short sequences, despite nearly normal levels of Ty1 RNA and VLPs. Our results suggest that the intramolecular interaction between the 5'-GAGGAGA and UCUCCUC sequences stabilizes an RNA structure required for efficient initiation of reverse transcription.
Figures

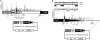
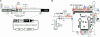


References
-
- Boeke, J.D. and Stoye, J.P. 1997. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. In Retroviruses (eds. H. Varmus), pp. 343–435. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. - PubMed
-
- Boeke, J.D., LaCroute, F., and Fink, G.R. 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet 197: 345–346. - PubMed
-
- Boeke, J.D., Garfinkel, D.J., Styles, C.A., and Fink, G.R. 1985. Ty elements transpose through an RNA intermediate. Cell 40: 491–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
