The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis
- PMID: 15663794
- PMCID: PMC548519
- DOI: 10.1186/1465-9921-6-11
The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis
Abstract
Background: Reactive oxygen species and tissue remodeling regulators, such as metalloproteinases (MMPs) and their inhibitors (TIMPs), are thought to be involved in the development of pulmonary fibrosis. We investigated these factors in the fibrotic response to bleomycin of p47phox -/- (KO) mice, deficient for ROS production through the NADPH-oxidase pathway.
Methods: Mice are administered by intranasal instillation of 0.1 mg bleomycin. Either 24 h or 14 days after, mice were anesthetized and underwent either bronchoalveolar lavage (BAL) or lung removal.
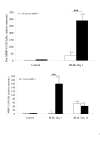
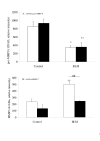
Results: BAL cells from bleomycin treated WT mice showed enhanced ROS production after PMA stimulation, whereas no change was observed with BAL cells from p47phox -/- mice. At day 1, the bleomycin-induced acute inflammatory response (increased neutrophil count and MMP-9 activity in the BAL fluid) was strikingly greater in KO than wild-type (WT) mice, while IL-6 levels increased significantly more in the latter. Hydroxyproline assays in the lung tissue 14 days after bleomycin administration revealed the absence of collagen deposition in the lungs of the KO mice, which had significantly lower hydroxyproline levels than the WT mice. The MMP-9/TIMP-1 ratio did not change at day 1 after bleomycin administration in WT mice, but increased significantly in the KO mice. By day 14, the ratio fell significantly from baseline in both strains, but more in the WT than KO strains.
Conclusions: These results suggest that NADPH-oxidase-derived ROS are essential to the development of pulmonary fibrosis. The absence of collagen deposition in KO mice seems to be associated with an elevated MMP-9/TIMP-1 ratio in the lungs. This finding highlights the importance of metalloproteinases and protease/anti-protease imbalances in pulmonary fibrosis.
Figures



References
-
- Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol. 1990;259:L159–184. - PubMed
-
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. - PubMed
-
- Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. - PubMed
-
- Ward PA, Hunninghake GW. Lung inflammation and fibrosis. Am J Respir Crit Care Med. 1998;157:S123–129. - PubMed
-
- Strausz J, Muller-Quernheim J, Steppling H, Nagel M, Ferlinz R. Oxygen radical production by alveolar macrophages in sarcoidosis in relation to activity status of bronchoalveolar lavage lymphocytes. Pneumologie. 1990;44(Suppl 1):222–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

