Genetical and functional investigation of fliC genes encoding flagellar serotype H4 in wildtype strains of Escherichia coli and in a laboratory E. coli K-12 strain expressing flagellar antigen type H48
- PMID: 15663798
- PMCID: PMC548302
- DOI: 10.1186/1471-2180-5-4
Genetical and functional investigation of fliC genes encoding flagellar serotype H4 in wildtype strains of Escherichia coli and in a laboratory E. coli K-12 strain expressing flagellar antigen type H48
Abstract
Background: Serotyping of O-(lipopolysaccharide) and H-(flagellar) antigens is a wideley used method for identification of pathogenic strains and clones of Escherichia coli. At present, 176 O- and 53 H-antigens are described for E. coli which occur in different combinations in the strains. The flagellar antigen H4 is widely present in E. coli strains of different O-serotypes and pathotypes and we have investigated the genetic relationship between H4 encoding fliC genes by PCR, nucleotide sequencing and expression studies.
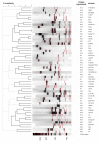
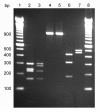
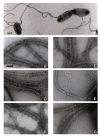
Results: The complete nucleotide sequence of fliC genes present in E. coli reference strains U9-41 (O2:K1:H4) and P12b (O15:H17) was determined and both were found 99.3% (1043 of 1050 nucleotides) identical in their coding sequence. A PCR/RFLP protocol was developed for typing of fliC-H4 strains and 88 E. coli strains reacting with H4 antiserum were investigated. Nucleotide sequencing of complete fliC genes of six E. coli strains which were selected based on serum agglutination titers, fliC-PCR genotyping and reference data revealed 96.6 to 100% identity on the amino acid level. The functional expression of flagellin encoded by fliC-H4 from strain U9-41 and from our strain P12b which is an H4 expressing variant type was investigated in the E. coli K-12 strain JM109 which encodes flagellar type H48. The fliC recombinant plasmid carrying JM109 strains reacted with both H4 and H48 specific antisera whereas JM109 reacted only with the H48 antiserum. By immunoelectron microscopy, we could show that the flagella made by the fliC-H4 recombinant plasmid carrying strain are constituted of H48 and H4 flagellins which are co-assembled into functional flagella.
Conclusion: The flagellar serotype H4 is encoded by closely related fliC genes present in serologically different types of E. coli strains which were isolated at different time periods and geographical locations. Our expression studies show for the first time, that flagellins of different molecular weigh are functionally expressed and coassembled in the same flagellar filament in E. coli.
Figures

References
-
- Drasar BS, Barrow PA. Intestinal Microbiology. In: Schlessinger D, editor. Aspects of Microbiology. Vol. 10. Washington D.C: American Society for Microbiology; 1985.
-
- Lior H. Classification of Escherichia coli. In: Gyles CL, editor. Escherichia coli in domestic animals and humans. Wallingford: CAB International; 1994. pp. 31–72.
-
- ∅rskov F, ∅rskov I. Methods in Microbiology. Vol. 14. London: Academic Press; 1984. Serotyping of Escherichia coli; pp. 43–112.
-
- Ewing WH. The Genus Escherichia. In: Ewing WH, editor. Edwards and Ewing's Identification of Enterobacteriaceae. New York: Elsevier Science Publishing Co; 1986. pp. 93–134.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

